SiteVault What's New in 25R3
Release Date: December 5, 2025
Enhancements to Creating Patients and Adding Participants
Users will find a streamlined workflow and redesigned interface when creating patients and adding participants to a study.
User Name Check when Creating Monitor/External Users
Administrators can enter the cross-domain user name in the email field when creating a new Monitor/External User. The system will search for the user name, retrieve the matching email address, and allow the administrator to proceed with a cross-domain account creation.
Extended Permissions for Site Staff
Add-On permissions have been relabeled to Extended Permissions and are expanded to include permissions that were previously reserved for Site Administrators. By assigning extended permissions to a site staff user (during user creation or editing), an administrator can delegate specific tasks for all studies at once. For example, assigning the Study Document Management extended permission allows a staff user to manage documents for every study in their SiteVault.
Relabeled Permissions
- Existing research organization permission Patients & Recruiting is relabeled to Patient & Participant Management.
- Existing site staff permission All Studies Schedule Builder is relabeled to Study Schedule Design & Management.
- Existing site staff permission All Studies Patients and Recruiting is relabeled to Patient & Participant Management.
Relabeled and Functionally Updated Existing Permissions
- Existing site staff permission All Studies Budgets and Contracts is relabeled to Financial Management and functions to enable staff to view and manage financial information across all studies.
- Existing site staff permission Site Profiles is relabeled to Site Document Management and functions to enable staff to view, upload, and manage site-level organization, product, and staff documents.
New Permissions
- New site staff permission Study Administrator grants edit access to all study data and settings, providing oversight across all studies.
- New site staff permission Study Document Management enables staff to view, upload, and manage study and study staff related documents across all studies.
Sponsor Field Not Required on Study
The Sponsor field is now optional when creating or editing a study record.
eBinder Document Viewer Enhancements
When viewing or classifying unclassified documents in the eBinder Document Viewer, the following document information is included in the Information panel:
- Version number in the document name header
- Version field
- Document lifecycle state
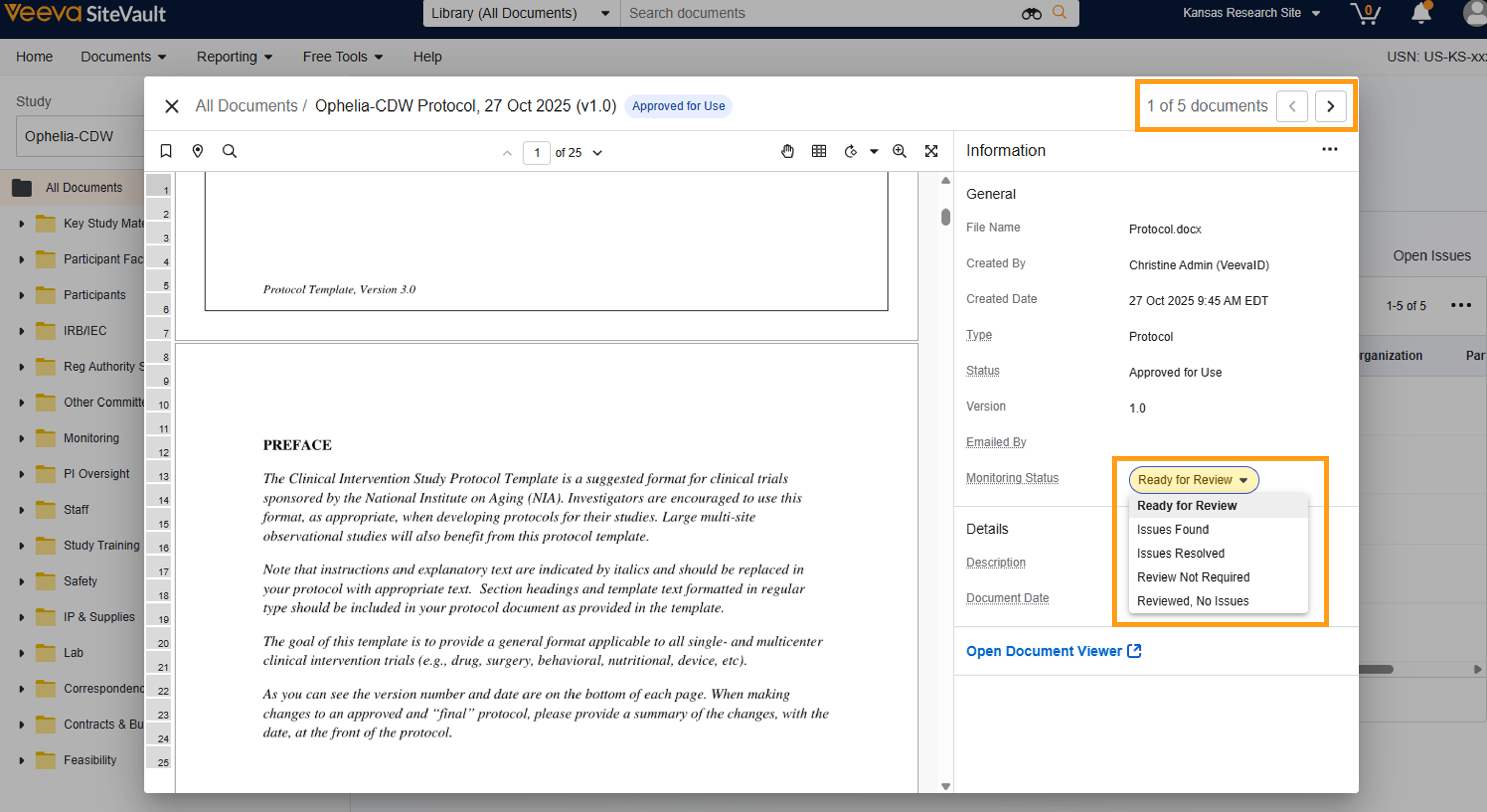
Monitoring: eBinder Document Viewer
The monitor review process is enhanced with the time-saving efficiencies offered by the Monitoring eBinder Document Viewer. This tool is an integrated viewer for monitors to review documents and update the Monitoring Status without leaving the Study eBinder. Selecting a document to review from the Study eBinder launches the Monitoring eBinder Document Viewer where monitors can page through the list of documents by selecting the onscreen or keyboard arrows. As documents are selected, the Information panel is updated with key document fields and tools, including options to update the Monitoring Status, view eSignature pages, or launch the document in the standard document viewer. When a document review is complete, monitors can remain in the viewer and simply select the arrow to navigate to the next document in the list. Document audit trails are updated with eBinder Document Viewer actions to capture when a document was viewed by a monitor.
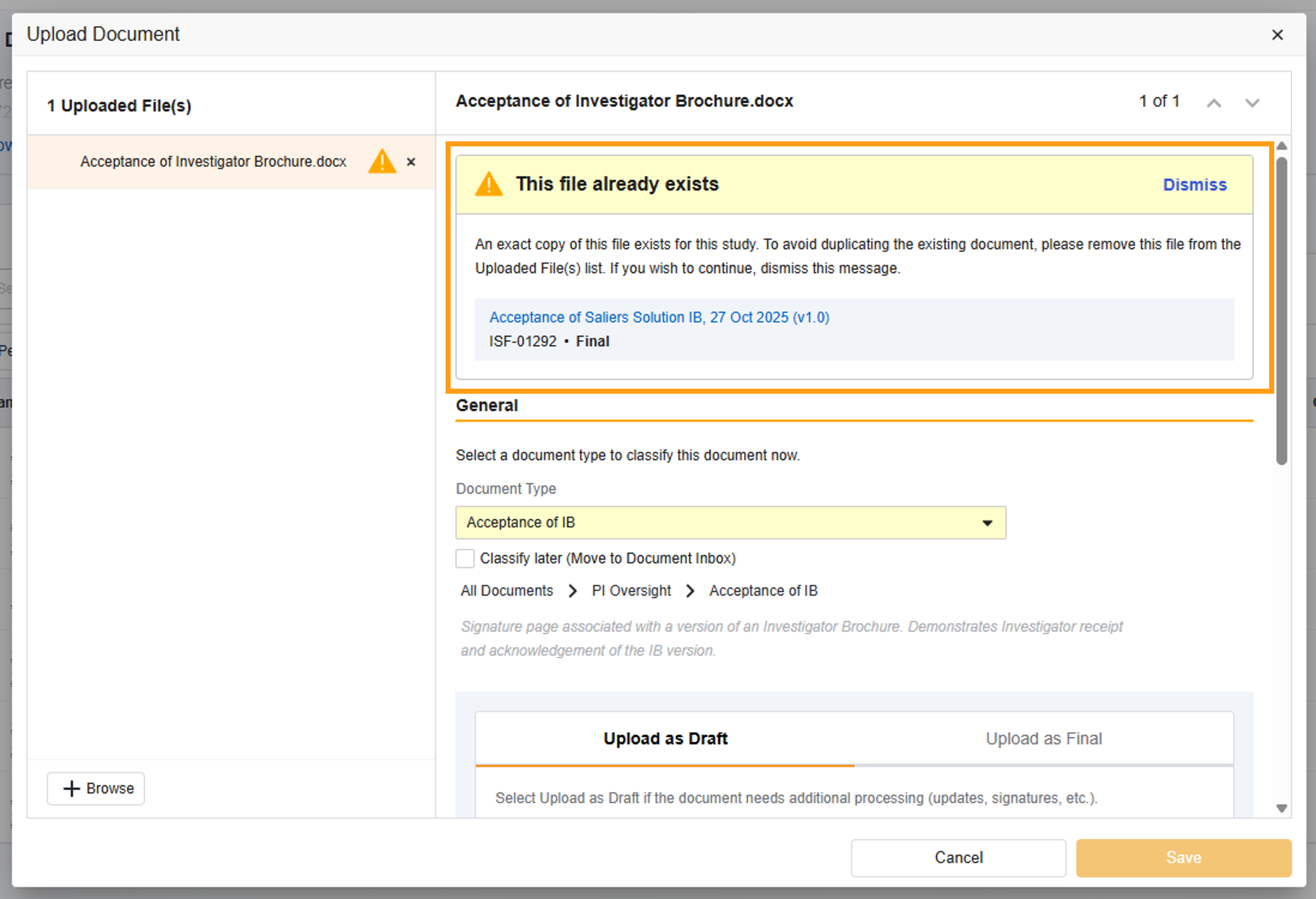
New Version Detection & Resolution
To simplify document management, this automated Study eBinder feature reduces the likelihood of uploading duplicate files into your eISF and streamlines the versioning process for all of your users.
Duplicate File Check (on all eISF Document Types): SiteVault will check if the source file being uploaded is an exact match to an existing document’s source file. If a match is found, you are presented with an alert advising you of the match and the option to cancel or proceed with the upload.
Document Type Check (on Specific eISF Document Types): SiteVault will check if the user-populated document fields (or metadata) match an existing document. If a match is found, you are presented with an alert advising you of the match and the option to upversion the existing document, cancel the upload of the document, or upload as a new, separate document.
Documents enabled with Document Type Check: Delegation of Authority, 1572 or Equivalent, Participant Enrollment Log, CV, Protocol, Lab Certification, Monitoring Visit Log, Medical License, Investigator Brochure, Informed Consent Form (blank), Training Evidence (non-study specific), Signature & Initials, IRB/IEC Composition, IRB/IEC Compliance.
Note:
- Checks occur only when a user uploads (and classifies) a document to the Study eBinder or classifies an unclassified existing document in the Study eBinder.
- Checks do not occur when creating a document in the Library.
- Checks do not occur when uploading an unclassified document to the Study eBinder Document Inbox.
- Checks do not occur on the following Site Document document types: Policy Memo, Standard Operating Procedures, Work Instructions.
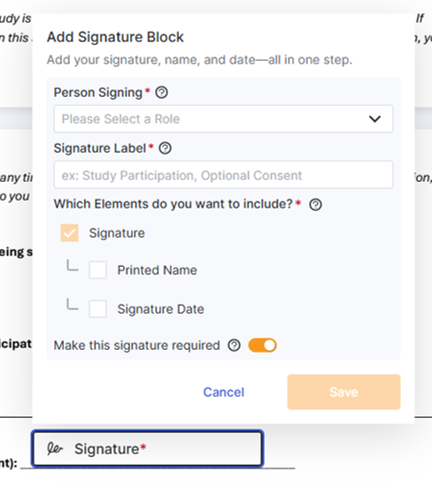
Multiple Signatures per Signatory
This feature allows one person to sign an eConsent in multiple places and introduces optional signature support. This update ensures every document is signed correctly while giving you the flexibility to handle complex forms simply.
Site User Impacts: When a consent form requires an individual to sign in more than one place, the editor can accommodate multiple signature fields for the same signatory within the same PDF document. To support this functionality, each signature element includes a required Signature Label field to capture the purpose of each unique signature, and a toggle field to note a signature as optional or required (default). The signature elements and options have been streamlined into a single element box. Also introduced with this feature is the eConsent status of Not Signed (optional) if a participant chooses not to complete an optional signature.
Patient Impacts: We've updated the signing experience to ensure patients and other signatories complete all necessary steps:
- No Missed Signatures: Signatories must scroll through and view all pages of the eConsent before applying any signatures. If a patient attempts to save a form without completing all required signatures, a warning is presented.
- Easier Navigation: Indicators in the page selector (on the side of the screen) inform patients if a signature on that page is required or optional. If a patient applies a signature but still has required signatures left on other pages, a banner appears. The page selector (right panel) is available to jump quickly to the incomplete signatures.
CTMS in SiteVault
We're excited to announce the full launch of SiteVault CTMS! CTMS is planned to be made generally available in SiteVault environments on January 6th.
Coverage Analysis
The Coverage Analysis feature is a study budget tool that, when enabled on the Budget Overview, allows users to precisely define billing responsibilities for each study activity as either Sponsor Paid, Routine Cost, or Not Billable on the Coverage Analysis tab. The tab also provides a space to capture activity-specific billing notes and Current Procedural Terminology (CPT) codes. Once designations and notes are saved, the system uses this information to update the Participant Fees table so that items designated as Routine Cost or Not Billable are not billed to the sponsor.
CTMS Reports Types
Users creating reports can select from the following CTMS report types:
- Billable Items
- Invoice
- Participant Visit
- Payment
- Study Schedule
Electronic Medical Record Connection
Site staff have the capability to create or update protocol and participant information by synchronizing data between CTMS and supported Electronic Medical Record (EMR) systems. This functionality reduces the need for manual, duplicative data entry across different systems and establishes a synchronized record to improve the consistency and accuracy of study and patient data. In 25R3, the EMR connection is available only to early adopters.
Finance Management - Billable Items
The following CTMS enhancements support users tasked with managing study finances.
- Users can create a custom billable item as a way to document billing adjustments (ex. pass-through costs, credits, and fee adjustments).
- Users are prevented from making changes to a billable item once it is associated with an invoice or payment.
- The Billable Items page includes a filter for the Invoiceable field.
Finance Management - Invoices
The following CTMS enhancements support users tasked with managing invoices.
- Users can change an invoice lifecycle status from Sent back to Draft.
- Invoice status Paid has been relabeled to Paid in Full.
- Invoice statuses Paid in Part and Paid in Full are updated automatically based on invoice activity.
The following fields are added to the Invoice page:
- Payment Terms (Net 30, Net 45, Net 60, Net 90, Other)
- Due date (a calculated field based on Invoice Date and Payment Terms)
- Purchase Order Number
Study Budget Enhancements
The following CTMS enhancements support users tasked with creating or editing Study Budgets.
- When creating site fees, overhead and withholding fields default to exempt but can be edited as needed.
- When viewing Participant Fees, base fees (x expected repetitions) are displayed instead of the base fee + overhead rate.
- Help text is available when hovering over the Invoiceable field.
Study Schedule - Arm Assignments
Arm Assignments allow schedule builders to associate specific arms of a study with different branches on the study's schedule. Users can create and name any number of study arms, then assign each to branches on the study's visit group diagram. After assigning arms to the schedule, users may filter the schedule and budget views, or add participants' visits by arm. Lastly, the budget will now display the total of participant fees associated with each arm of the study, providing a summation of all required fees a site will accrue should a participant complete all visits.
Study Schedule Enhancements
The following CTMS enhancements support users tasked with building or editing study schedules.
- The character limit on visit names is increased.
- Visits may be moved between visit groups.
- Offset Early and Offset Late fields support zeros in addition to null values. This functionality is intended to support asymmetrical offsets (i.e. +0/-.
- The presentation of fields in the Visits tab provides additional clarity around visits, target dates, and offset days. Users will find the visit components streamlined together to create a flow more conducive to the scheduling process.
Study Schedule - Visits
The following CTMS enhancements support users tasked with building and managing study schedule visit groups.
- When repeating is enabled, the repeating icon is displayed next to the visit group name in the schedule grid.
- The limitation on the amount of repetitions available for a repeating visit group has been removed.
- Repeat Maximum field label is relabeled to Maximum Repetitions.
- Repeat Default field label is relabeled to Default Repetitions.
- The Cycle Length field is to help users define the designed interval or spacing between each instance of the repeating visit group, typically as specified in the study protocol. When a visit group is marked as repeatable, the Cycle Length field defines the length for each repetition, which is used to calculate the future visit dates and their associated offsets. If a Visit Group represents cycles starting later than cycle 1, the starting cycle number may be specified in the Starting Cycle Number field for the purposes of labeling participant visits with the correct cycle.
Working with Patients on CTMS Studies
The following CTMS enhancements support users managing participant scheduling and visits.
- When marking a visit as Completed, the calendar will pre-select the visit's scheduled date, if one is available.
- Repeating visits will include the visit iteration after the visit name once the visit is added to the participant schedule. For example, if a second screening visit is added to a participant’s schedule, it will appear as Screening (2).
No release notes match the selected filters.