Release Highlights for Sites
Release Date: December 5, 2025
The 25R3.0 release of Veeva products for sites is available December 5, 2025. Summaries of the major highlights are below. To view the full release notes for a product, follow the link in the product's section, if applicable.
SiteVault
Select here to read a full list of what's new in SiteVault this release.
Extended Permissions for Site Staff
Add-On permissions have been relabeled to Extended Permissions and are expanded to include permissions that were previously reserved for Site Administrators. By assigning extended permissions to a site staff user (during user creation or editing), an administrator can delegate specific tasks for all studies at once. For example, assigning the Study Document Management extended permission allows a staff user to manage documents for every study in their SiteVault.
Relabeled Permissions
- Existing research organization permission Patients & Recruiting is relabeled to Patient & Participant Management.
- Existing site staff permission All Studies Schedule Builder is relabeled to Study Schedule Design & Management.
- Existing site staff permission All Studies Patients and Recruiting is relabeled to Patient & Participant Management.
Relabeled and Functionally Updated Existing Permissions
- Existing site staff permission All Studies Budgets and Contracts is relabeled to Financial Management and functions to enable staff to view and manage financial information across all studies.
- Existing site staff permission Site Profiles is relabeled to Site Document Management and functions to enable staff to view, upload, and manage site-level organization, product, and staff documents.
New Permissions
- New site staff permission Study Administrator grants edit access to all study data and settings, providing oversight across all studies.
- New site staff permission Study Document Management enables staff to view, upload, and manage study and study staff related documents across all studies.
eBinder Document Viewer Enhancements
When viewing or classifying unclassified documents in the eBinder Document Viewer, the following document information is included in the Information panel:
- Version number in the document name header
- Version field
- Document lifecycle state
New Version Detection & Resolution
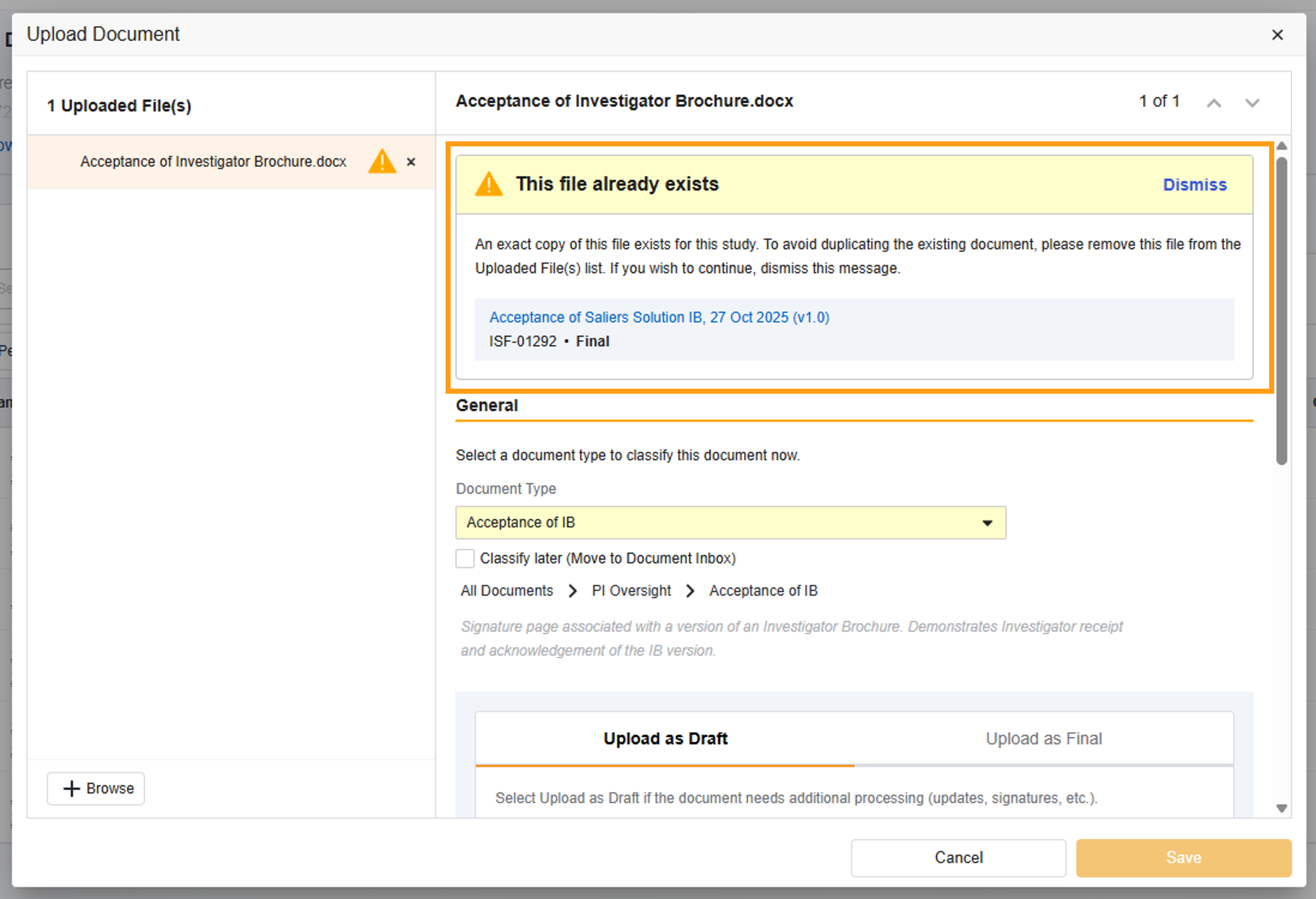
To simplify document management, this automated Study eBinder feature reduces the likelihood of uploading duplicate files into your eISF and streamlines the versioning process for all of your users.
Duplicate File Check (on all eISF Document Types): SiteVault will check if the source file being uploaded is an exact match to an existing document’s source file. If a match is found, you are presented with an alert advising you of the match and the option to cancel or proceed with the upload.
Document Type Check (on Specific eISF Document Types): SiteVault will check if the user-populated document fields (or metadata) match an existing document. If a match is found, you are presented with an alert advising you of the match and the option to upversion the existing document, cancel the upload of the document, or upload as a new, separate document.
Documents enabled with Document Type Check: Delegation of Authority, 1572 or Equivalent, Participant Enrollment Log, CV, Protocol, Lab Certification, Monitoring Visit Log, Medical License, Investigator Brochure, Informed Consent Form (blank), Training Evidence (non-study specific), Signature & Initials, IRB/IEC Composition, IRB/IEC Compliance.
Note:
- Checks occur only when a user uploads (and classifies) a document to the Study eBinder or classifies an unclassified existing document in the Study eBinder.
- Checks do not occur when creating a document in the Library.
- Checks do not occur when uploading an unclassified document to the Study eBinder Document Inbox.
- Checks do not occur on the following Site Document document types: Policy Memo, Standard Operating Procedures, Work Instructions.
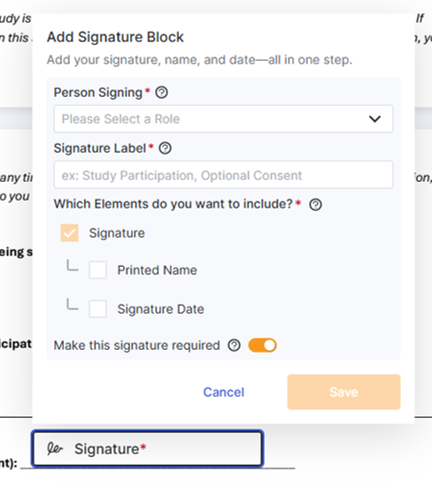
Multiple Signatures per Signatory
This feature allows one person to sign an eConsent in multiple places and introduces optional signature support. This update ensures every document is signed correctly while giving you the flexibility to handle complex forms simply.
Site User Impacts: When a consent form requires an individual to sign in more than one place, the editor can accommodate multiple signature fields for the same signatory within the same PDF document. To support this functionality, each signature element includes a required Signature Label field to capture the purpose of each unique signature, and a toggle field to note a signature as optional or required (default). The signature elements and options have been streamlined into a single element box. Also introduced with this feature is the eConsent status of Not Signed (optional) if a participant chooses not to complete an optional signature.
Patient Impacts: We've updated the signing experience to ensure patients and other signatories complete all necessary steps:
- No Missed Signatures: Signatories must scroll through and view all pages of the eConsent before applying any signatures. If a patient attempts to save a form without completing all required signatures, a warning is presented.
- Easier Navigation: Indicators in the page selector (on the side of the screen) inform patients if a signature on that page is required or optional. If a patient applies a signature but still has required signatures left on other pages, a banner appears. The page selector (right panel) is available to jump quickly to the incomplete signatures.
Veeva eCOA
Select here to read a full list of what's new in Veeva eCOA this release.
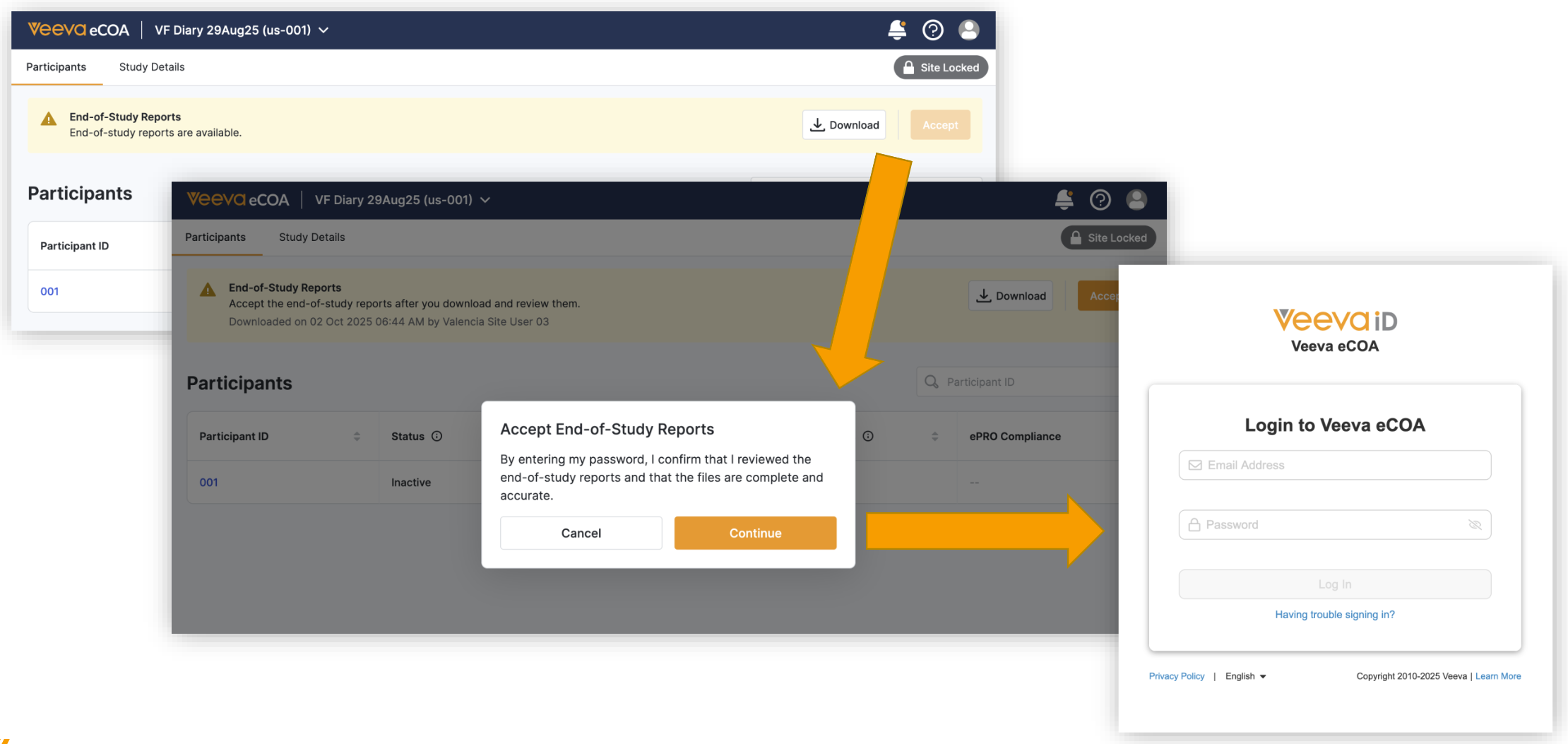
End-of-Study Media Distribution and Reporting
You can access, download, and accept end-of-study reports sent by your sponsor.
To accept the media, you provide an electronic signature using your Veeva ID login information.
Improved Survey Compliance Updates for Event Datetime Changes
When you update an event datetime, associated surveys are updated to better show compliance.
Missed, Available, and Scheduled surveys are canceled, and new instances are created when needed. Completed surveys are unchanged. The system also displays information about what will happen to surveys in the list when an event datetime is changed, which gives you more control and visibility over the process.
When all surveys for an event are marked as Missed, a banner is displayed to let you know that you can update the event's datetime. This can help you correct events that have an incorrect date added.
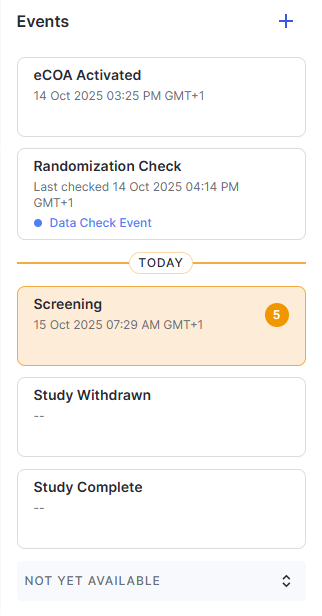
Event Lists Sort by Date and Time
Events lists are now sorted by date and time to show the most relevant actions first.
The eCOA Activated event is listed at the top, followed by past events and today's events (with an icon next to it to help you see where the current date is in the list).
Future events and events with no set date are then shown in the order the sponsor/CRO configured.
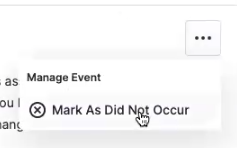
Mark Events as Did Not Occur
You can mark scheduled events as Did Not Occur from the event list.
You can only use this option for events that have no surveys in a final state, and you have to provide a reason for the audit trail.
Veeva EDC
Select here to read a full list of what's new in Veeva EDC this release.
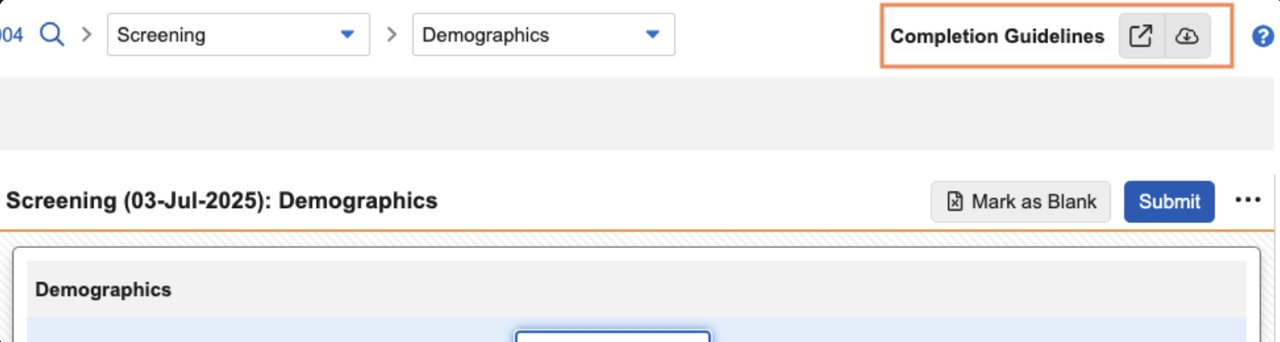
EDC Completion Guidelines
Sponsors now have the option to instantly share data entry instructions to guide site users. Site users can access the file from any screen within the study, in the top right corner.
DICOM Pixel Masking
Site Users can easily protect patient privacy using granular controls to redact any PHI/PII found directly on an image. This update is automatically available in studies using Veeva EDC Imaging.
Improved UI for Grid Views During Auto-save
As Site users rapidly enter data in repeating items, the data they enter will be more visible as it auto-saves. While adding and editing items within the grid view, the value entered will appear grayed out (uneditable) as it auto-saves.
Veeva RTSM
Site User Audit Access
As part of our-site centric approach to helping site users to have access to their data as they need it, the ability to view RTSM audit data is accessible through the user interface. On an as-needed basis, a blinded site user can view and export blinded audit data. Users can choose from a single kit or subject, or over a certain time-period, up to a full blinded view of all data changes associated with their site.
Veeva Site Connect
Select here to read a full list of what's new in Veeva Site Connect this release.
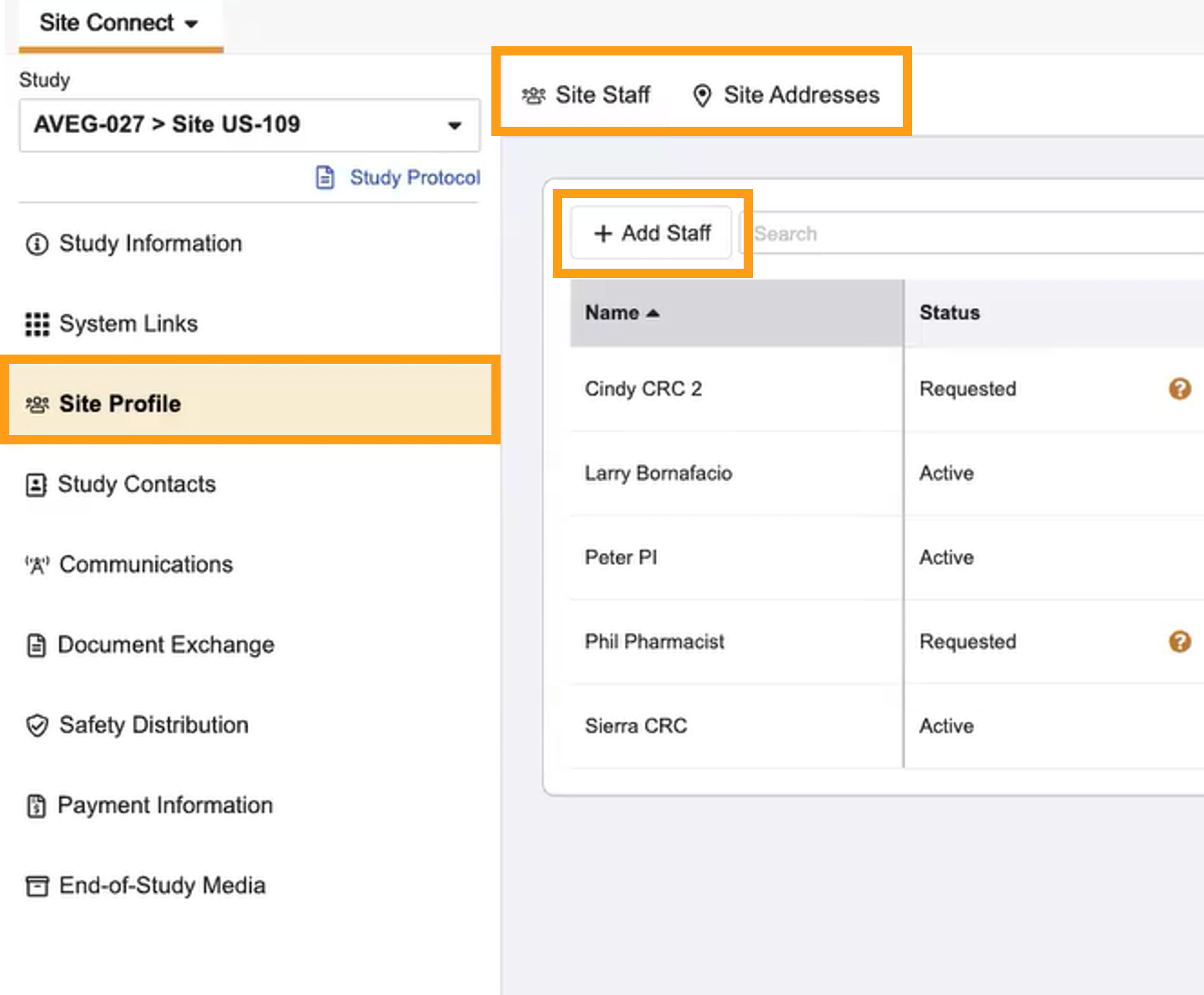
Site Staff in Site Connect
This feature empowers sites to manage their own site staff within Site Connect. Sites can view the staff tracked by the Sponsor/CRO and request changes to the staff list as appropriate, without external communication. Site Staff actions are available from the Site Staff tab under Site Profiles.
- Site users can add new site staff or edit existing staff Start and End Dates, Roles, and Responsibilities.
- When a site user creates or updates staff details, the Sponsor/CRO receives a request to approve or reject the change.
- The site can view the request status updated in real time in Site Connect.
- If approved, the user is granted access to Site Connect on the requested start date.
- If rejected, the site user who initiated the change receives a notification with the rejection reason.
- Navigation Updates include:
- Site Profiles now houses Site Staff and Site Addresses.
- Study Admin is relabeled to Site Settings and is at the bottom of the Site Connect menu.
- Study Contacts is a new option on the Site Connect menu.
Remember Site Staff Roles & Responsibilities
To provide a more streamlined user experience for site users adding other site staff to a study, the system retains the Role and Responsibilities field values that were initially selected and associates those values with the site staff. Going forward, when the site staff is added again, the remembered role and responsibilities will be automatically populated. These fields can be modified if needed.
File to SiteVault Duplicate Document Detection
With this feature, the system now checks for existing document versions in SiteVault when a user performs the File to SiteVault action, thereby avoiding duplicates from being created.
When a user selects the File to SiteVault action from an individual document and a duplicate version is detected, the system will present a confirmation dialog, providing the user with information about the existing document in SiteVault. From this dialog, the user can choose to cancel the filing to avoid creating a duplicate or proceed with filing the document to SiteVault. In instances where documents are filed to SiteVault in bulk, duplicate versions will not be filed if detected.
This enhancement ensures data integrity, saves site users time and effort by avoiding unnecessary data duplication and cleanup, and provides users with control over how to handle potential duplicates.
Site Front Door in VeevaID
The VeevaID homepage (id.veeva.com) list of studies has been updated to clearly display which studies users have access to in each Sponsor/CRO Vault. When a study is selected, users are navigated to the selected study's System Links tab in Site Connect. Previously, users could access individual systems from the VeevaID homepage, but there was no indicator of a study's sponsor or CRO. This enhancement provides a single gateway for site users to access their studies with more visibility of the Sponsor/CRO-to-study connection.
Veeva Study Training
Select here to read a full list of what's new in Veeva Study Training this release.
Display Latest Steady State Documents on Learner Task Page
With this feature, Learners can view the latest Steady State document related to a completed Training Assignment in the History tab. Previously, when accessing documents through the History tab, a superseded document would cause Learners to encounter a “Document Not Found” error.
This feature provides Learners the ability to look back at previously completed training and access the current related document. This can be used as a way to quickly access the Library directly from assignments that the Learner knows they have already completed.
- Vault presents the Learner with the latest Steady State document.
- A message is displayed telling the user which document version they actually trained on, as well as the version they are currently viewing.
Training Homepages: Export Functionality
This feature enables Learners to export data from the Open and History tab as a CSV document. Previously, the History tab could only be exported as a PDF. These documents can also be exported from the My Study Team page by the Learner’s Manager.
- The data exported is based on the current page filters.
- From My Study Teams, the assignments of the Open, Completed, and All tabs can be exported to CSV.
My Learning Tab: Announcements
A new Announcements area is being added to the My Learning page. Customers can use it as a communication channel for training-focused messaging, helping to deliver important internal campaigns or compliance reminders directly where Learners engage.
Announcements appear as cards at the top of the My Learning page. Up to ten active announcements rotate every 20 seconds. Learners can manually navigate between announcements or jump to specific ones. Learners may hide the announcement area until their next log-in.
Veeva Training Enhancements
This feature includes a grouping of small enhancements to accessibility (color contrast), Instructor-Led Training, Curriculum Outcomes permissions, Substitute Rules, and minor security updates. See the Quality and Training release notes in Vault Help for more details.
VeevaID
Enhanced Multi-Factor Authentication
VeevaID users will now be prompted for Multi-Factor Authentication (MFA) based on the user’s country, in addition to the existing MFA behavior in which Vault prompts for MFA once every 30 days.
Specifically, VeevaID will additionally initiate MFA when:
- A VeevaID user logs in from a new country
- A VeevaID user has two successful login attempts from two different countries within four hours
This enhancement increases the security measures for VeevaID by leveraging the existing MFA functionality in additional risky circumstances.
Site Front Door in VeevaID
This feature allows site users to view the list of Site Connect studies they have access to in each Sponsor/CRO Vault via the VeevaID homepage (id.veeva.com). Previously, site users could access individual systems from the VeevaID homepage, but there was no knowledge of which studies were being run in which Vaults. Selecting a study will take the site to the System Links tab in Site Connect for that study.
With this streamlined approach, this feature serves as a single gateway for site users to access all Site Connect studies in one place.
No release notes match the selected filters.