Veeva eCOA What's New in 25R3
Release Date: December 5, 2025
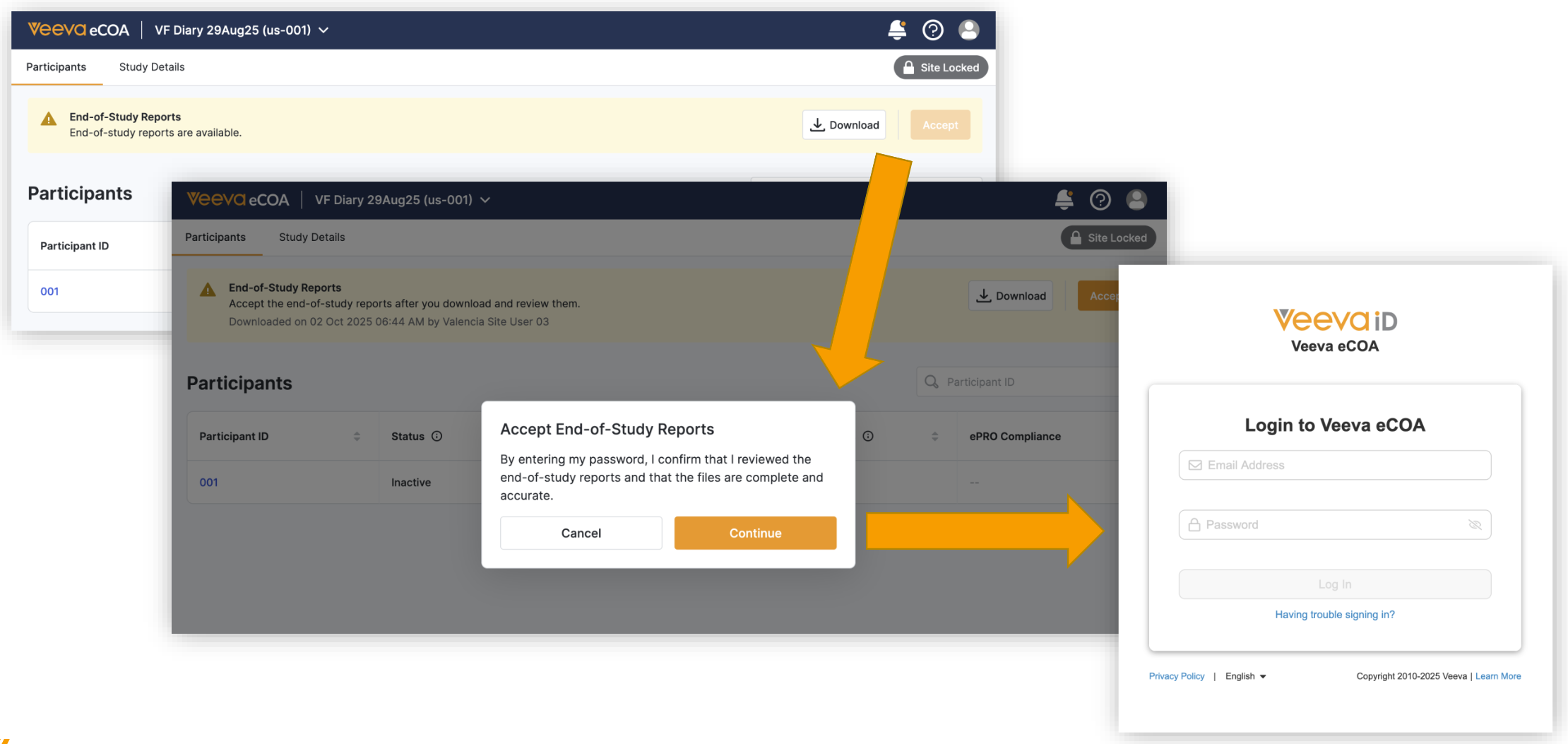
End-of-Study Media Distribution and Reporting
You can access, download, and accept end-of-study reports sent by your sponsor.
To accept the media, you provide an electronic signature using your Veeva ID login information.
Improved Survey Compliance Updates for Event Datetime Changes
When you update an event datetime, associated surveys are updated to better show compliance.
Missed, Available, and Scheduled surveys are canceled, and new instances are created when needed. Completed surveys are unchanged. The system also displays information about what will happen to surveys in the list when an event datetime is changed, which gives you more control and visibility over the process.
When all surveys for an event are marked as Missed, a banner is displayed to let you know that you can update the event's datetime. This can help you correct events that have an incorrect date added.
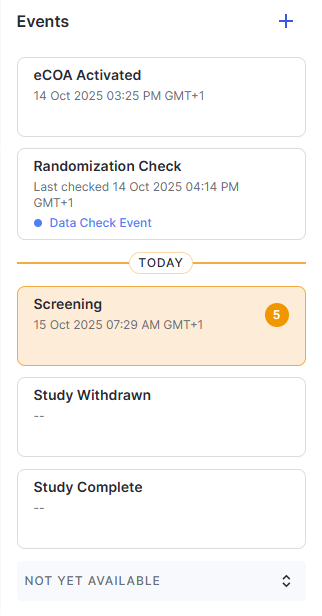
Event Lists Sort by Date and Time
Events lists are now sorted by date and time to show the most relevant actions first.
The eCOA Activated event is listed at the top, followed by past events and today's events (with an icon next to it to help you see where the current date is in the list).
Future events and events with no set date are then shown in the order the sponsor/CRO configured.
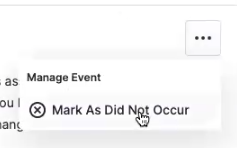
Mark Events as Did Not Occur
You can mark scheduled events as Did Not Occur from the event list.
You can only use this option for events that have no surveys in a final state, and you have to provide a reason for the audit trail.
Keeping the Original 'eCOA Activated' Date for Reactivated Participants
When you reactivate a Participant, the system restores them to Active status but does not change the eCOA Activated event date.
This ensures the date shown always reflects the participant's first activation in the study.
Notification Emails Language Changes
This feature updates email notifications to only be sent in the following supported site languages, based on the country that the Sponsor/CRO configured for your study site:
- Italy: Italian
- Japan: Japanese
- China: Chinese (Simplified)
- All Other Countries: English
Improved Veeva ID Navigation
You can now more easily navigate to Veeva ID from anywhere in Veeva eCOA to manage account settings or switch between systems.
General Usability and UI/UX Enhancements
This update enhances eCOA to work better on touch screen devices.
Conditional and Populated Events
Events can be configured to only become available when you reach a specific point in the study.
An event may only become available after the previous event has been started, or it may only become available when a criteria check's requirements have been met.
Events can also be configured to be automatically populated from data collected in a survey.
When events are configured this way, you can set the initial date, but once populated by another source, you cannot update the date yourself.
This automation reduces your administrative work by simplifying the event sequence.
Surveys Scheduled by Other Surveys
Surveys can be configured to be scheduled based on the completion of another survey, instead of waiting for a fixed event date.
This update helps reduce the amount of manual work you have to do to keep the study running smoothly.
If a survey is configured to be automatically scheduled, you cannot change when it becomes available in eCOA.
Automatically Populating Participant and Event Groups from Other Systems
Your sponsor/CRO can now configure Event Datetimes and Participant Groups to be automatically filled in from other external clinical systems.
This means you cannot manually edit or add values for these groups and events in eCOA once they are created by the other system, ensuring data accuracy.
Veeva eCOA Single Sign-On Support
Your sponsor/CRO can now configure Single sign-on (SSO) for a study.
This allows you to log in to Veeva eCOA with your sponsor's existing company network credentials.
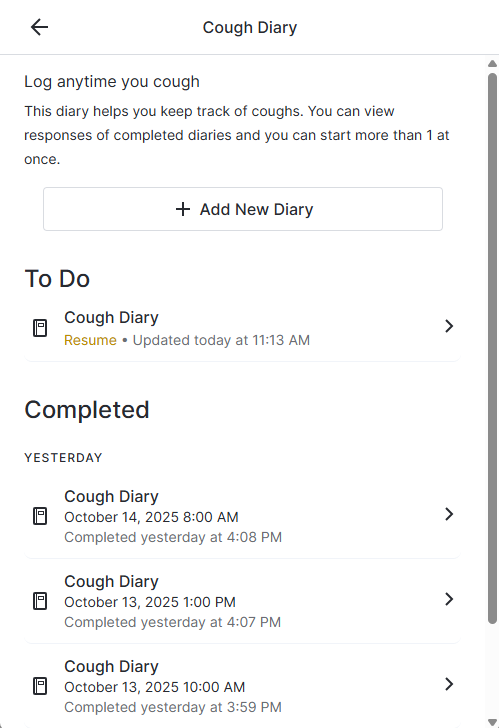
New Survey Component: Diary
Studies can be configured to include Diaries in the As-Needed section on a participant's To Do page.
When a participant selects a diary, it opens a page where the user can add a new diary entry, continue an in-progress entry, or view completed entries (when configured by the sponsor).
Participants can have multiple diary entries in progress at one time.
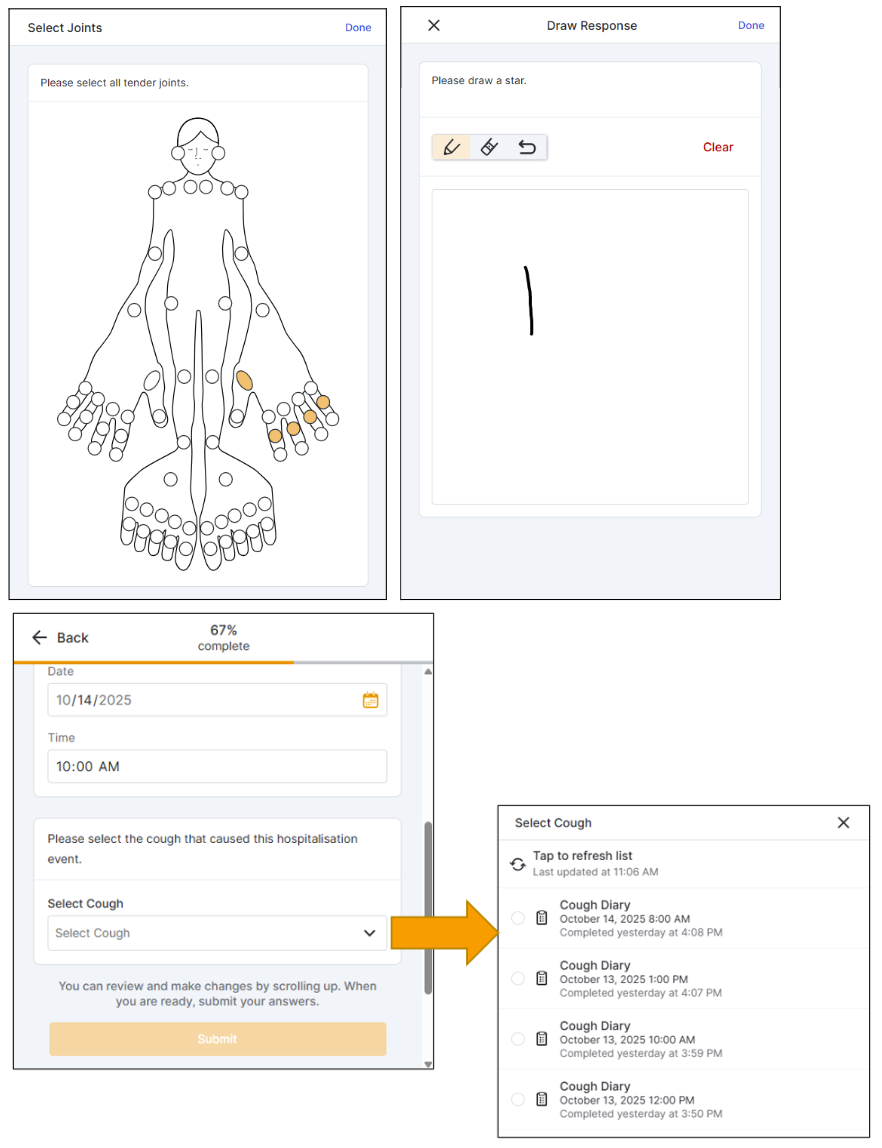
New Survey Question Types: Survey-to-Survey Linking, Joint Count, and Free Draw
Site Staff or Participants can now answer survey questions with new responses:
- Survey-to-Survey Linking: This question type allows users to select from a list of their previously submitted surveys.
- Joint Count Image: This question type allows users to select specific joints on an anatomical image to answer a question.
- Free Draw Image: This question type allows users to draw a picture as a survey response.
No release notes match the selected filters.