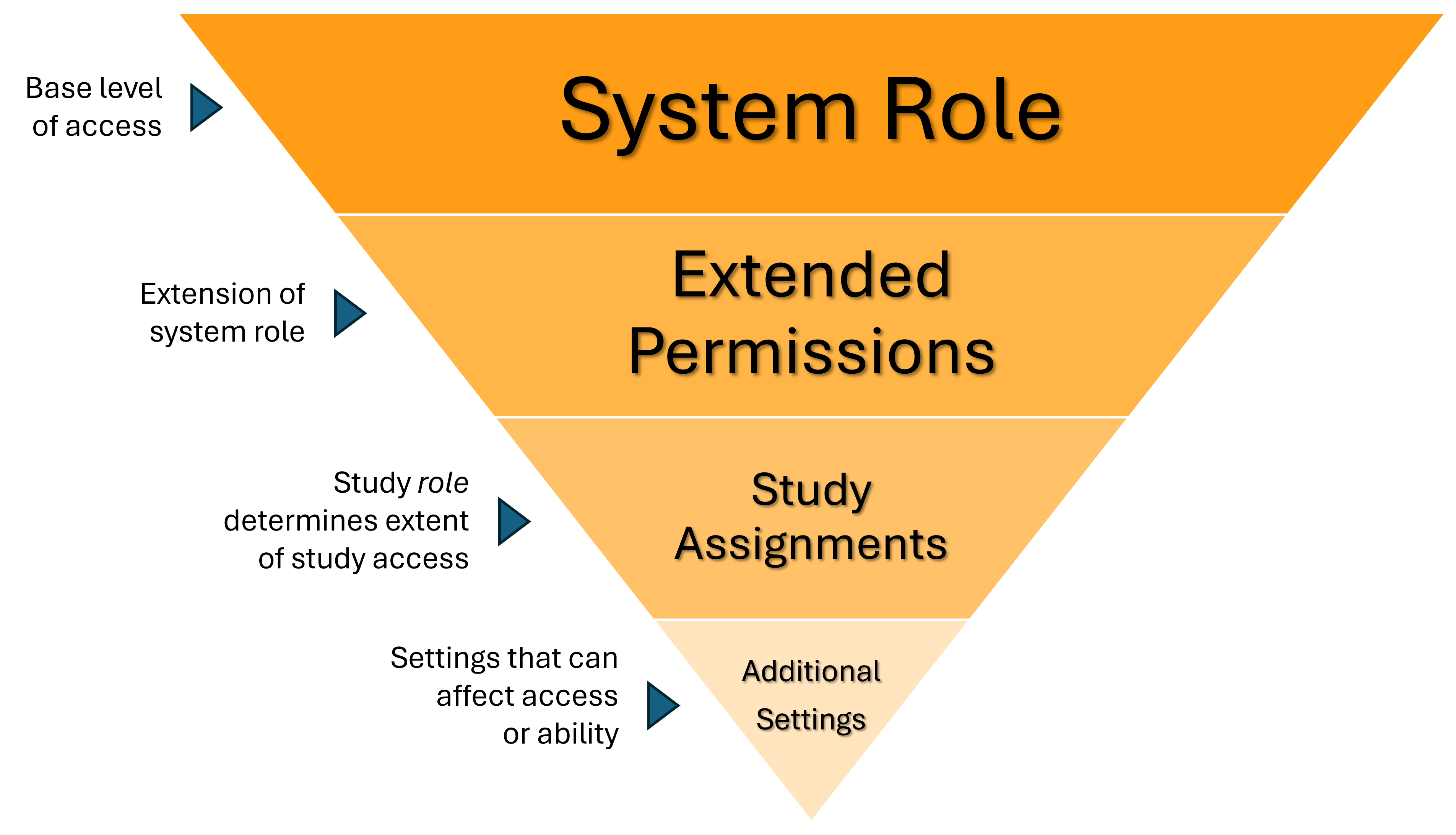
SiteVault’s system of roles and permissions defines what a user can see and do within the platform. This page introduces the different levels of access, from the foundational System Role to specific Extended Permissions and Study Assignments. These elements work together with other settings to ensure users have the right level of access to documents and data.
For additional access guidance, download the SiteVault Security Matrix or SiteVault User Access Guide.
Sites vs. Research Organizations: The Importance of User Context
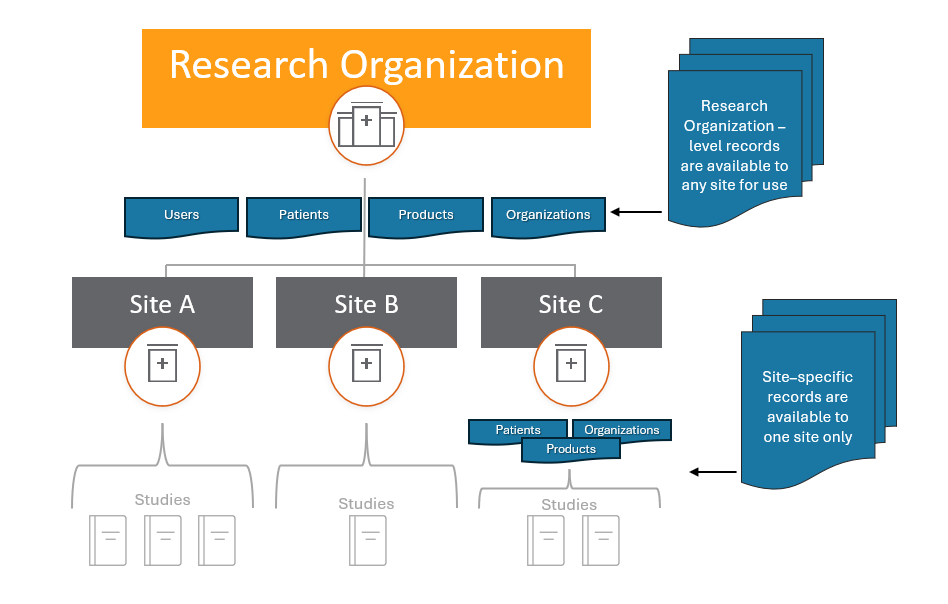
SiteVault is structured to accommodate a single research site seeking a tool to organize its study materials or a larger organization requiring multiple SiteVault environments. Research organizations have multiple sites (or divisions/departments), each managing individual SiteVault environments to which the organization’s SiteVault provides oversight. Users have system roles at the research organization and site levels. Users requiring access at multiple sites can have different system roles at different sites.
Records for patients, products, or organizations, can be shared across all sites within an organization.
System Roles
System roles define the base level of access at a site or research organization. System roles are assigned when an administrator creates a user account. Based on a user’s responsibilities across sites, a user may have different roles at different sites.
| Level | Role | Description |
| Research Organization | Research Organization Administrator |
|
| Research Organization Staff |
|
|
| Research Organization External |
|
|
| Site | Site Administrator |
|
| Site Staff |
|
|
| Site External |
|
|
| Site Viewer (all studies) |
|
Extended Permissions
Extended permissions add flexibility to a user’s system role by granting additional access. These permissions are assigned when an administrator creates or edits a user account.
By assigning extended permissions to a Site Staff user, an administrator can delegate specific tasks for all studies at once. For example, assigning the Study Document Management extended permission allows a staff user to manage documents for every study in the system, and assigning Study Schedule Design & Management allows them to build schedules for all studies.
Note: Administrators can create and manage user system roles and extended permissions; these user administration tasks are not included in any extended permissions.
| Level | Permission | Description |
| Research Organization | Patient & Participant Management | Create/manage patient and participant data (including recruitment) across the research organization and its sites |
| Site | Study Administration | Create/manage studies, study data, and settings across all studies |
| Study Document Management | Create/manage study and study staff-related documents across all studies | |
| Site Document Management | Create/manage site-level documentation for staff, IRB/IECs, labs, sponsors, and products across all studies | |
| Patient & Participant Management | Create/manage patient and participant data (including recruitment) across all studies | |
| Study Schedule Design & Management (CTMS) | Create/manage CTMS study schedule components across all studies | |
| Financial Management (CTMS) | Create/manage CTMS study budget and manage financial information across all studies |
Study Assignments
A study assignment gives a user access to a specific study and its documents. Users can only be assigned one Study Role per study.
| Level | Roles |
| External | Auditor/Inspector |
| Sponsor/CRO | |
| Site Staff | Clinical Research Coordinator |
| Data Coordinator | |
| Budgets & Contracts | |
| Principal Investigator | |
| Regulatory Coordinator | |
| Research Nurse | |
| Subinvestigator | |
| Pharmacist | |
| Other Non-Investigator |
Additional Settings that Affect User Access and Permissions
Something as simple as a document’s lifecycle state can determine user visibility, but SiteVault also offers several more granular settings that provide an additional level of secure flexibility.
Restricted Document Visibility
Study-level option for site staff and external users, granted in study assignments
The Restricted document functionality allows sites to limit document visibility to specific users assigned to the study. The feature is intended to be used for blinded/masked studies where certain documents (ex. randomization information) must remain hidden from the larger study staff. Restricting documents and staff is a two-step process that includes first marking individual study documents as Restricted and then granting individual study staff members access to restricted documents. Only study staff members with access to restricted documents can view study documents marked as Restricted.
External Users Granted Access to Site Documents
Site-level option for external users
A Monitor or an External User may request to access your site’s business process documents, such as standard operating procedures (SOPs), work instructions, or policy memos. These are stored in the Site eBinder, which is not visible to non-site staff users. However, you can share these documents with Monitors/External Users on an individual document basis. This is not a user setting; it is a document setting that is set through a user action. When you choose to share a document, the document is visible to all Monitors and External Users at your site.
Accept and Complete Monitoring Issue Tasks
Study-level option for site staff users, granted in study assignments
When creating a study team assignment an administrator (or site staff with the Study Management extended permission) can select the Monitoring Issue Recipient option to designate the user as responsible for reviewing monitoring issue notifications and completing any related tasks.