Release Considerations Veeva ePRO is planned to be generally available in 23R1. Future release dates and information are subject to change.
About the Release
Important Dates
The release dates below are important for the Digital Trials Platform. See the About the 23R1 Release page in the Vault Release Notes for Vault release dates.
| Date | Event | Description |
|---|---|---|
| March 27 | Pre-Release | The release is deployed to pre-release Vaults and the MyVeeva for Patients pre-release environments |
| March 27 - April 21 | Validation Package |
The Digital Trials Platform validation package is available in VeevaDocs on the dates below. The Digital Trials Platform validation package includes validation documents for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO.
|
| April 21 | General Release | The release is deployed to all general release Vault PODs and MyVeeva for Patients general release environments. |
Release Information and Impacts
The release resources and information below are planned to be available on the dates in the Publication History column. See the About the 23R1 Release page in the Vault Release Notes for Vault resources and information.
| Resource | Description | Publication History |
|---|---|---|
| Digital Trials Platform 23R1 Release Impact Assessment |
The release impact assessment analyzes the regulatory, configuration, and user impacts of the MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO features in this release. |
Updated March 8 |
| What's New |
The What's New information provides detailed explanations of the new features for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO. |
Updated April 21 |
| Fixed Issues |
The MyVeeva for Patients and SiteVault Fixed Issues lists at the bottom of this page list issues that affected previous versions or the pre-release and are fixed in this general release. |
Both published March 31 |
| Known Issues |
See the following pages for known issues that affect this general release or previous ones and aren't fixed yet: |
Both published March 31 |
| Maintenance Releases |
See the following pages for fixed issues that affected the general release: |
Both available with the first maintenance release |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new features across the Digital Trials Platform:
|
See links |
What’s New
MyVeeva for Patients Mobile
Mobile App Biometrics
This feature allows MyVeeva users to use their biometric information (such as face scan or fingerprint scan) to log in to the Android or iOS app and submit signed eConsent forms. After a user sets up their biometric information, the system requires them to verify their identity every thirty days by entering a multifactor authentication code.
For more information, see the following help topics:
- Account Settings in MyVeeva for Patients Help
- Consent Forms in MyVeeva for Patients Help

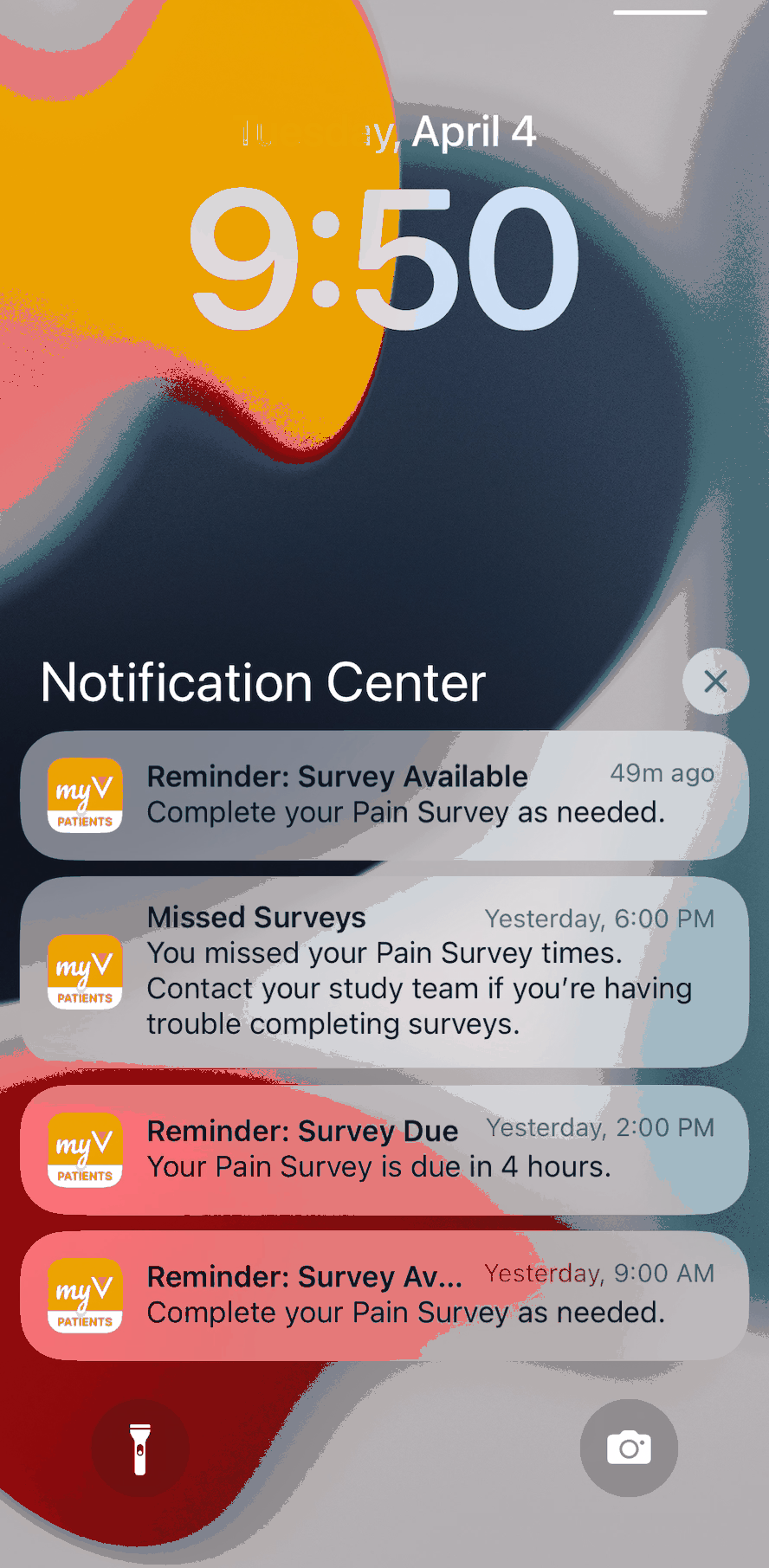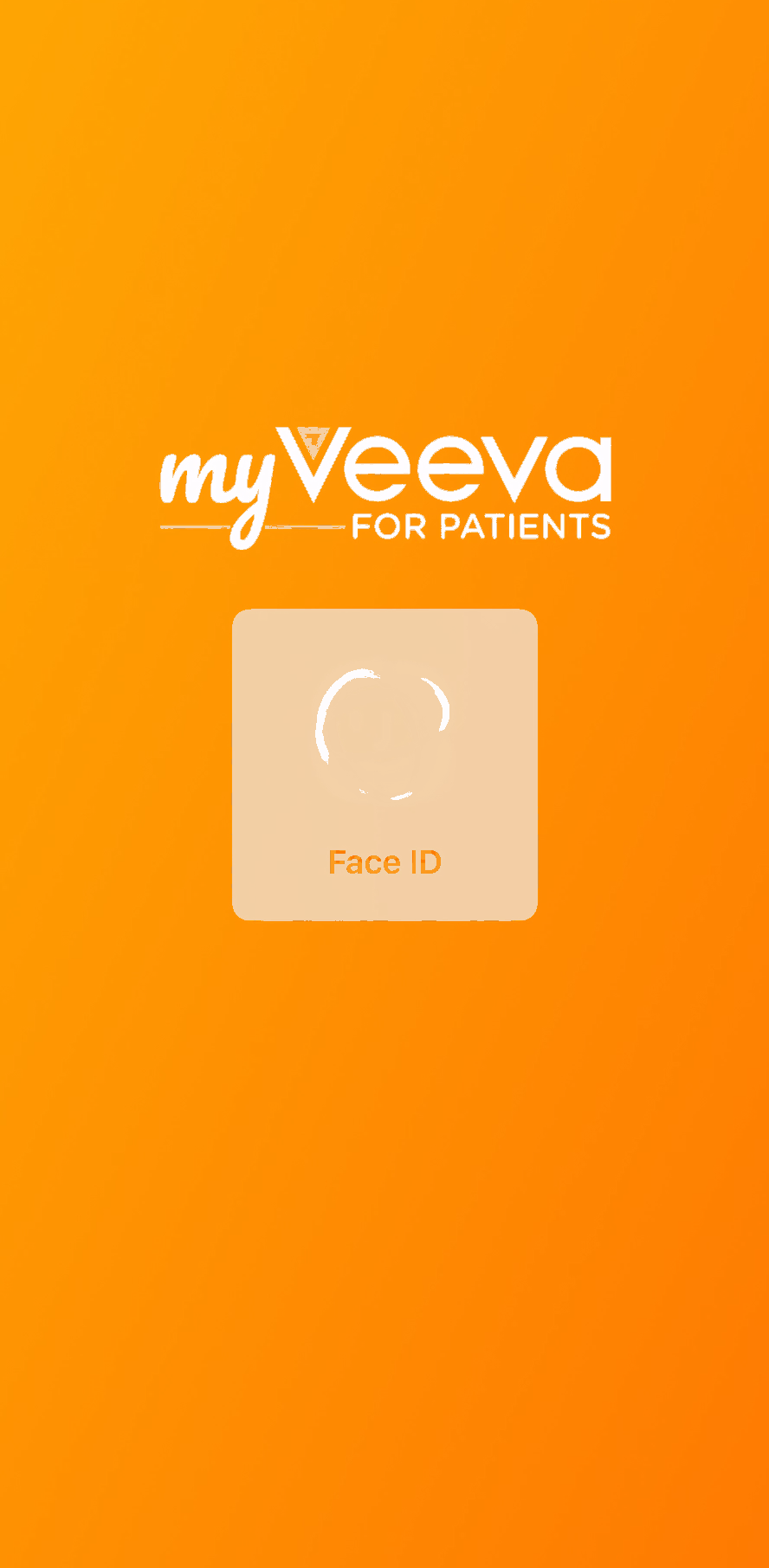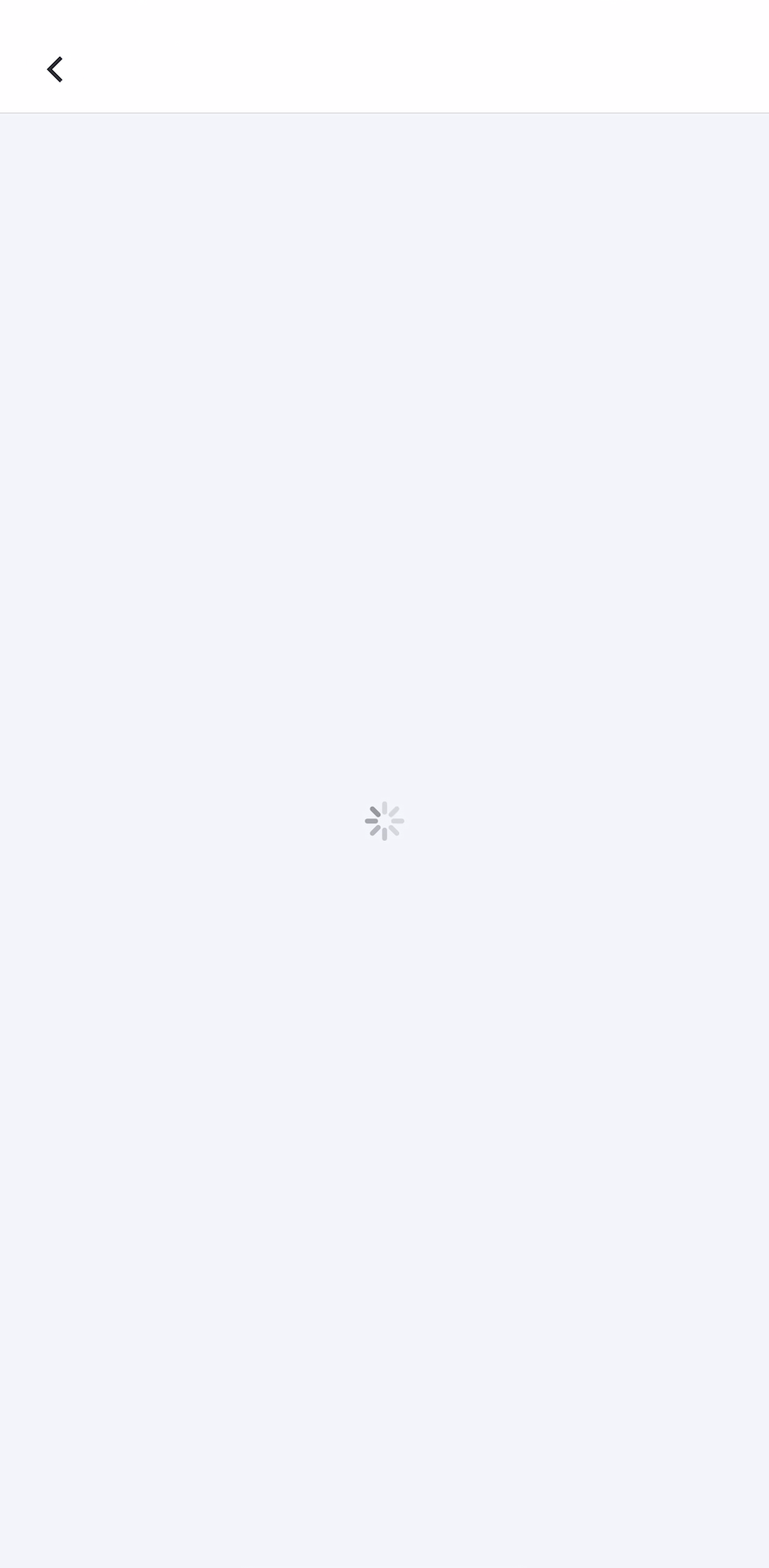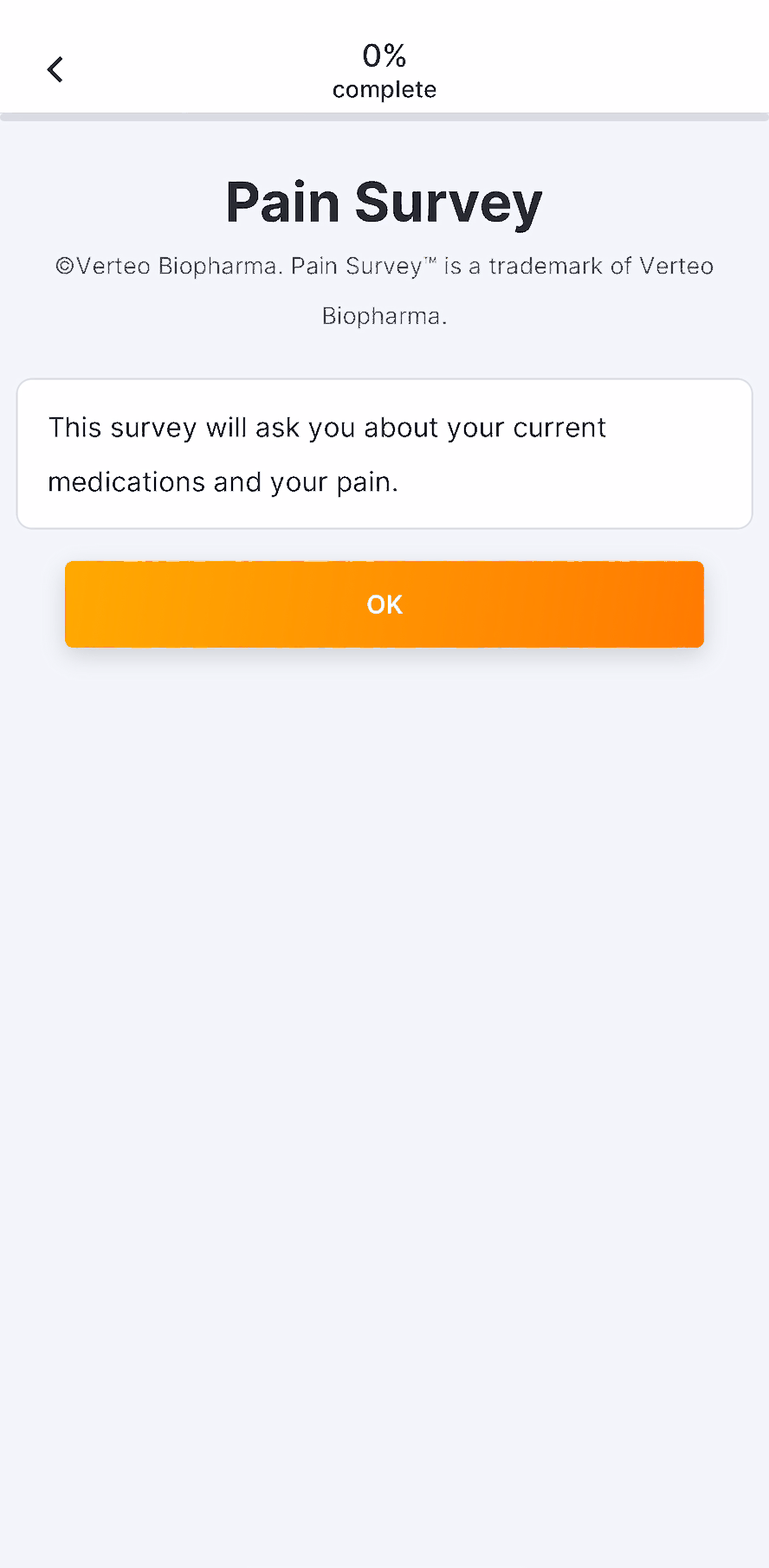
Android and iOS Push Notification Enhancements
This feature enhances mobile push notifications for MyVeeva users who have the Android or iOS app installed on their device. Selecting a mobile push notification now navigates a user to the task or area of the app related to the notification. When a user receives multiple push notifications, they’re grouped by type in their device’s notification center.
For more information, see Getting Started in MyVeeva for Patients Help.
Download Android or iOS App From Mobile Web App
This feature allows MyVeeva users to be prompted to download the Android or iOS app when they use the mobile web app.
For more information, see Getting Started in MyVeeva for Patients Help.
MyVeeva for Patients Platform
Visits
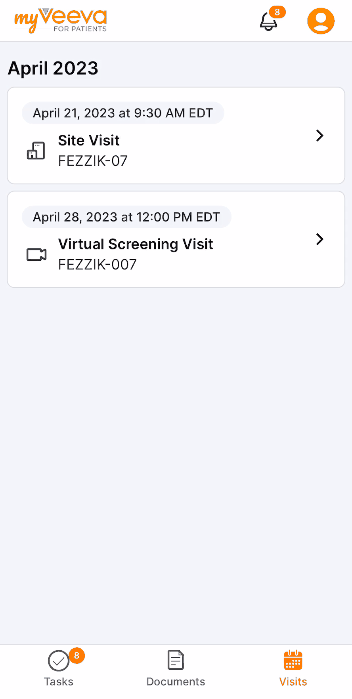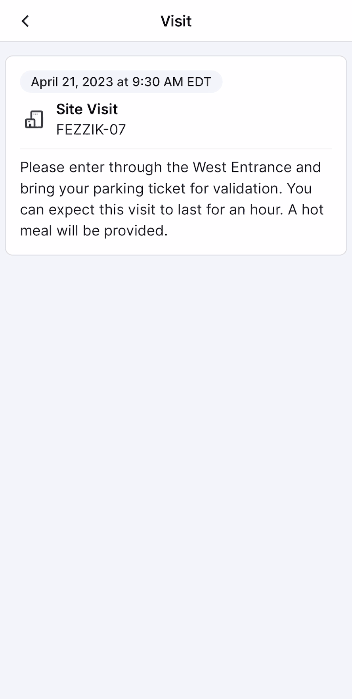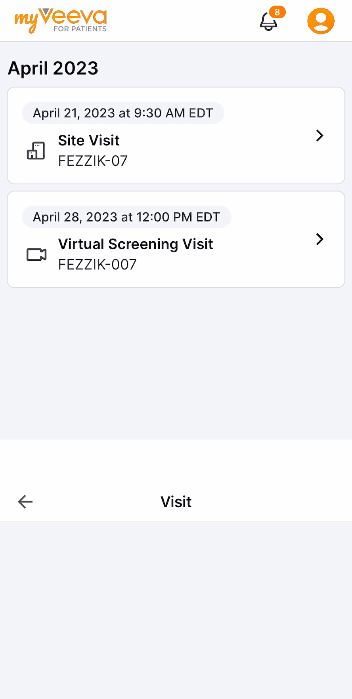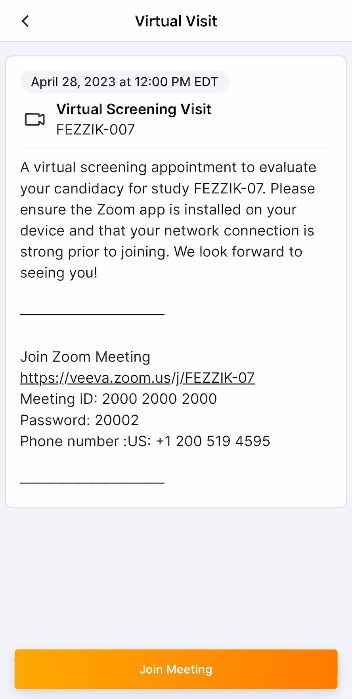
This feature allows site users to schedule study visits and virtual study visits for MyVeeva users. Users can copy a participant’s MyVeeva calendar alias in SiteVault and schedule an appointment in Outlook or Google Calendar with the alias as a recipient/attendee. The meeting’s details, date, and time appear as a visit on a MyVeeva user’s Visits page. If a meeting invitation contains a virtual meeting link from Meet, Skype, Teams, or Zoom, the meeting appears as a virtual visit that MyVeeva users can join from the MyVeeva for Patients app. If site users edit the meeting’s date, time, or details or cancel the meeting on their Outlook or Google calendar, the changes apply to the MyVeeva user’s scheduled visit. Site users can invite multiple MyVeeva users to the same calendar meeting to create a visit for each invitee without revealing the list of recipients to any MyVeeva user.
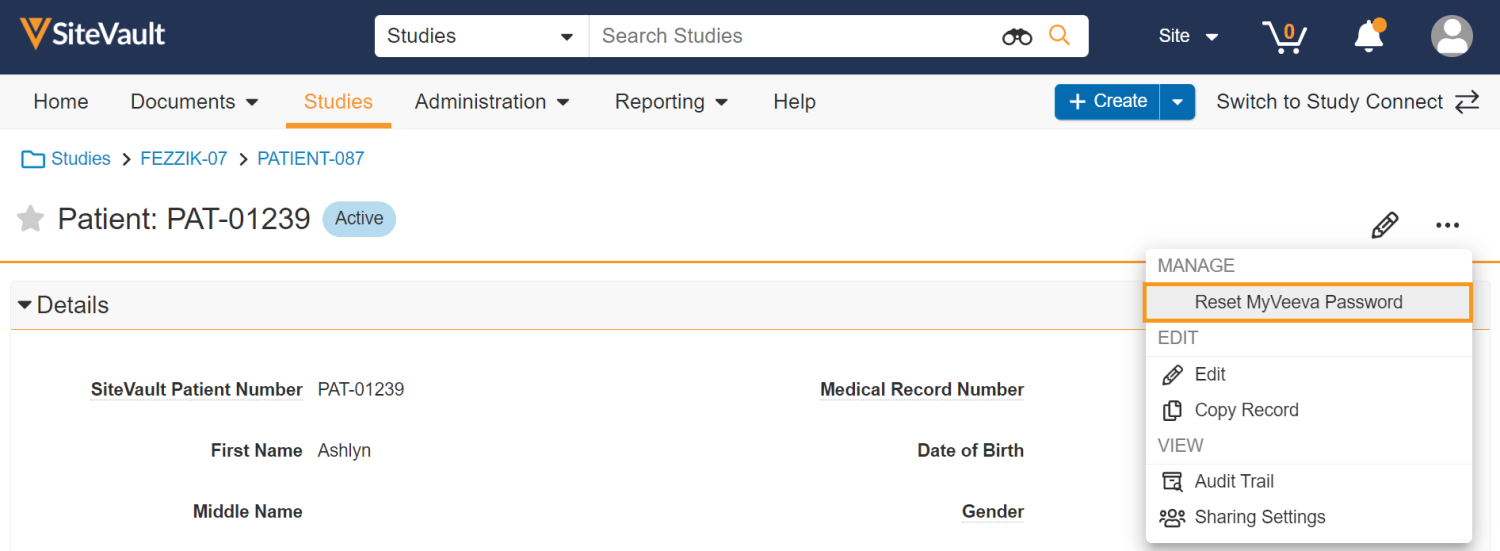
Allow Site User to Reset MyVeeva User’s Password
This feature allows site users to send a password reset email to a MyVeeva user if they forget their MyVeeva for Patients password and no longer have access to their phone number on file. Site users can send a password reset email from the patient profile in SiteVault.
Prevent Further Changes to Participant’s Patient ID in SiteVault
This feature prevents site users from changing a study participant’s Patient ID in SiteVault after a study participant is invited to use MyVeeva for Patients. A participant’s Patient ID locks immediately after site users create a participant if a study has study documents that SiteVault automatically shares.
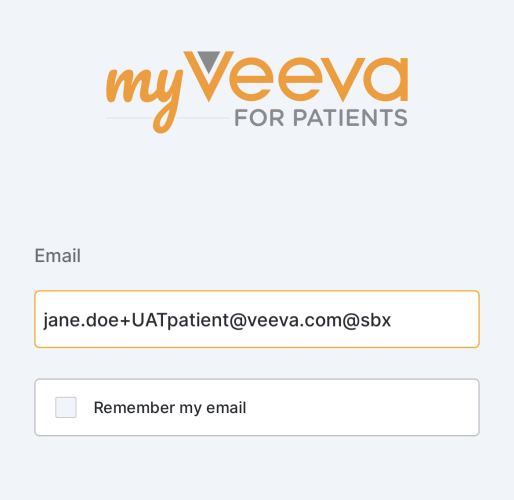
Simplified MyVeeva for Patients Sandbox Environment Login
This feature simplifies logging in to a MyVeeva for Patients sandbox environment for sponsor users regardless of their geographic location or client of choice (Android, iOS, or web browser). Users on a browser can go to https://patients-sbx.myveeva.com/ to log in to the sandbox environment, and Android and iOS users can enter “@sbx” after their username to log in to the sandbox environment. Users who bookmarked the previous web URL or updated their iOS app settings to log in to the patient-sbx-us environment may need to update those bookmarks and settings.
For more information, see Performing User Acceptance Testing (UAT) on an ePRO Collection in Clinical Vault Help.
SiteVault Release Webinar Recording
SiteVault Release Highlights
SiteVault eReg
Single Action for Document Status Change and Certify as Copy
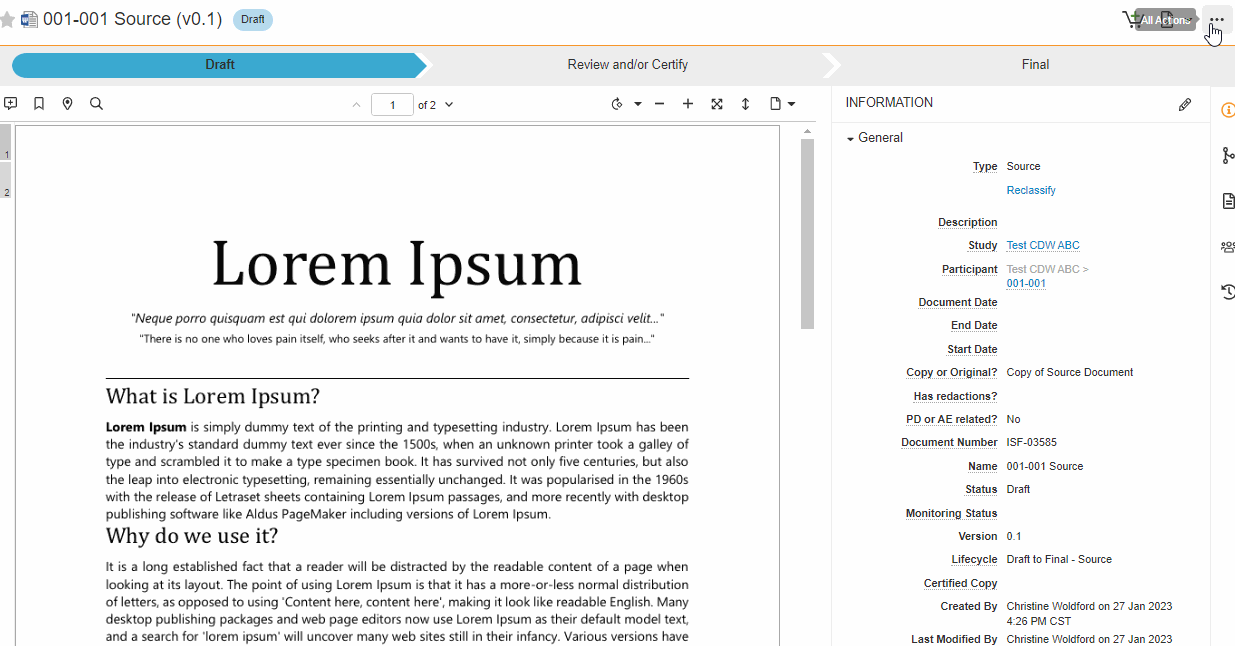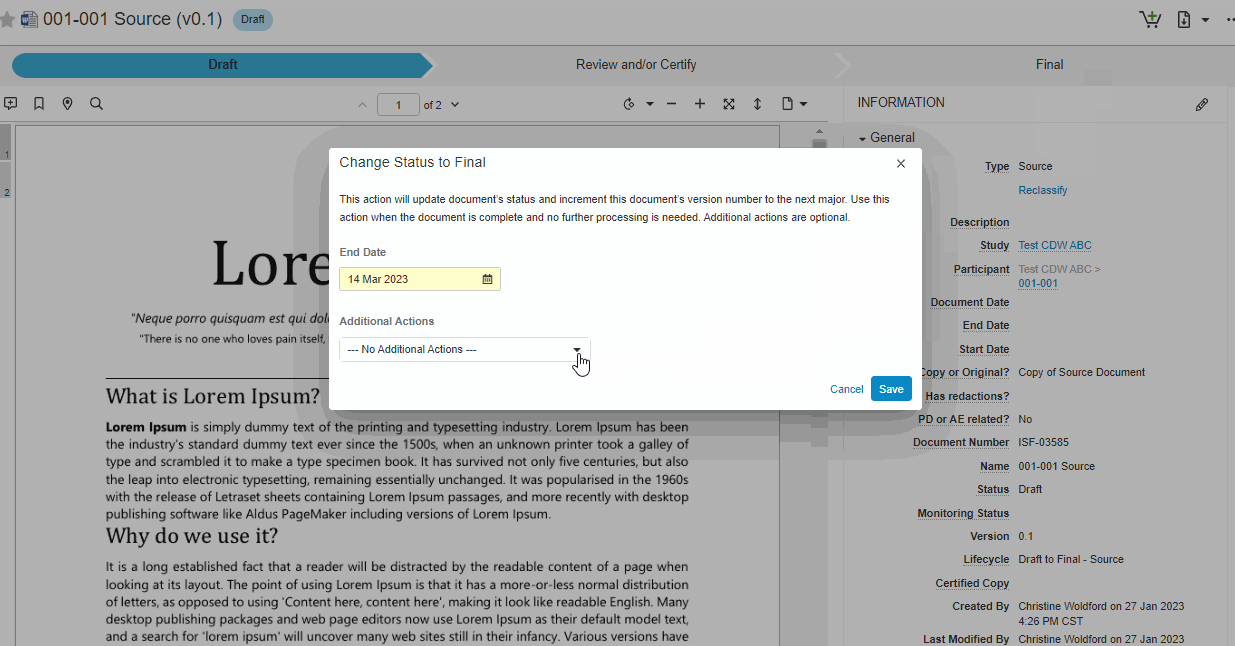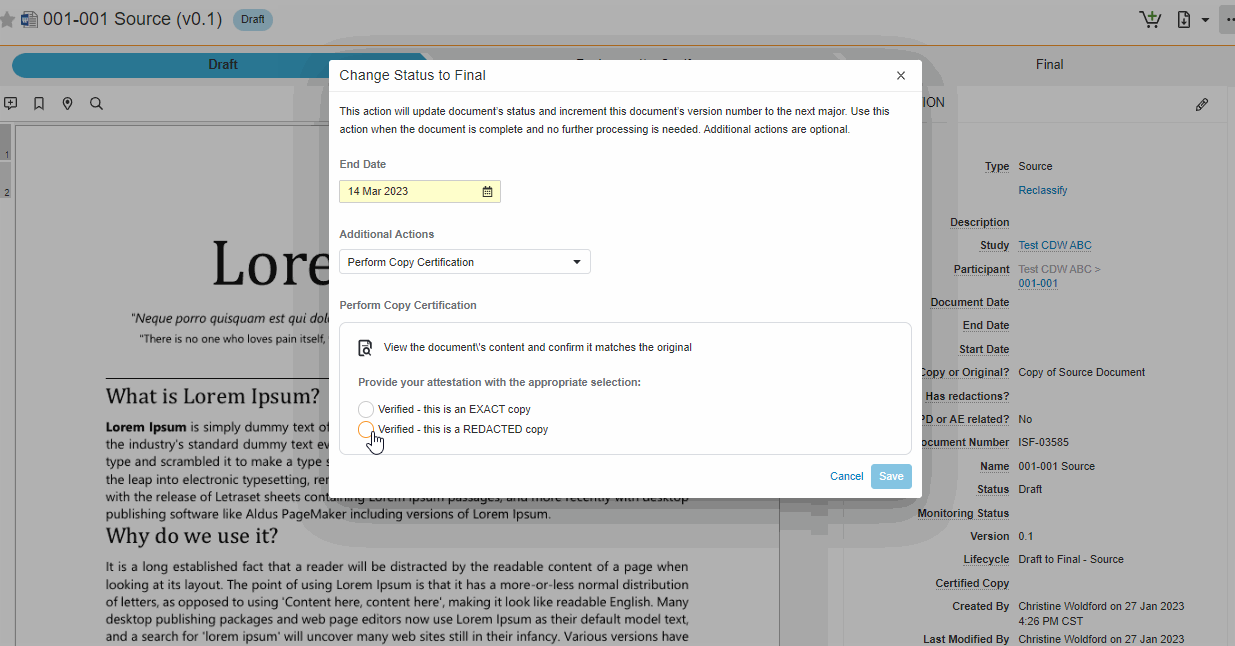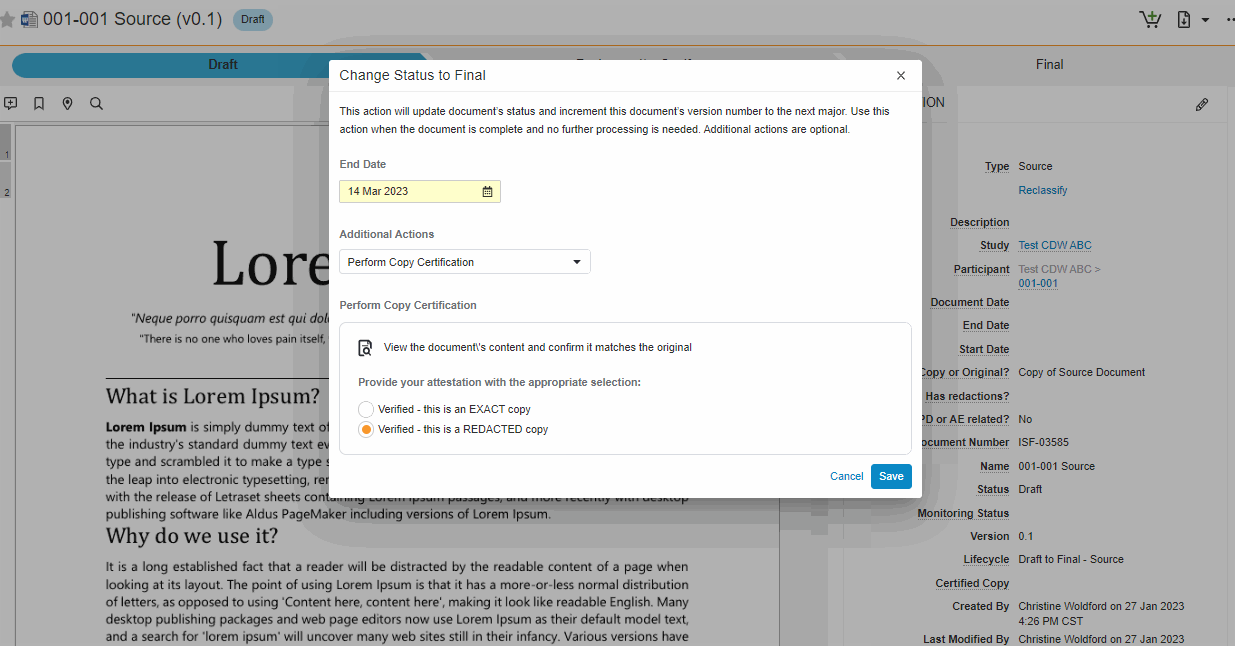
This feature brings consistency to finalizing documents across all document types in SiteVault eReg. We’ve replaced the Change State to (Final State), Finalize Source, and Certify as Copy workflows with a single all-in-one action. The new all-in-one document actions give users the ability to change the status of a document and perform the copy certification process in one easy flow.
Note This new action can be accessed under the Document or All Actions menus.
Study eBinder Enhancements
This feature increases eBinder functionality and ease of use:
- Drag and Drop Documents into Study eBinder Document Grid
With this feature, you can upload a document to the Study eBinder by dragging a file into the document grid (table).
- Folders with Document Indicators
With this feature, eBinder will include a document indicator and a document count to help you easily identify which folders contain documents.

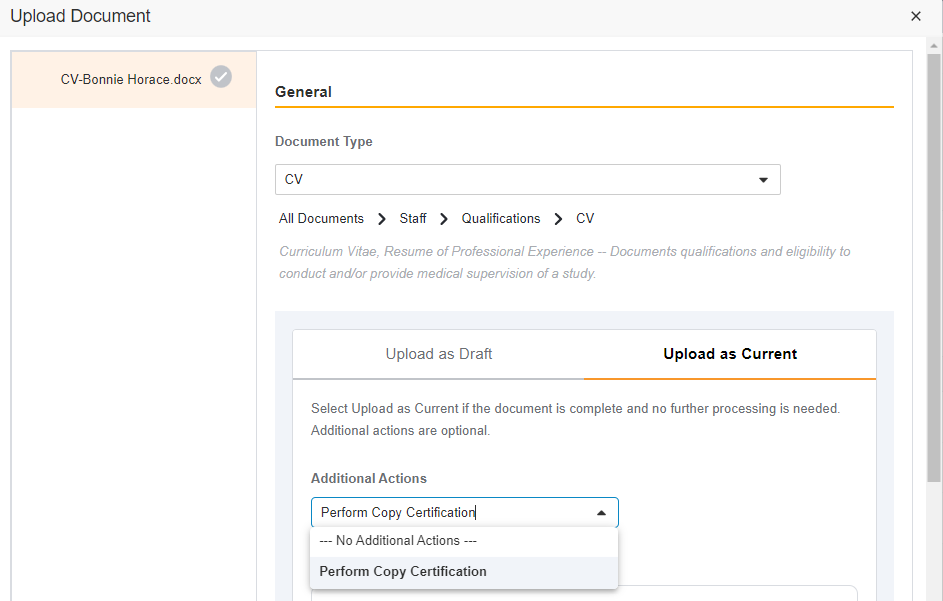
- eBinder Upload to Current/Approved/Final State
This feature gives you the ability to upload documents directly into their steady state.
Document Workflow Enhancements
This feature offers new document workflows and a streamlined process for existing document workflows.
- Generic Review and eSignature workflows
This feature introduces two standard workflows:
- Review: The Review workflow allows you to request others to review a document and provide feedback by using the annotation tool. The Review workflow is available on the following lifecycles: Draft to Approved for Use, Draft to Current, and Draft to Final.
- eSignature Approval Workflow: eSignature Approval allows you to collect eSignatures on documents requiring one or more signatures for approval. The eSignature Approval workflow is available on the following lifecycles: Draft to Current and Draft to Final.
- Review: The Review workflow allows you to request others to review a document and provide feedback by using the annotation tool. The Review workflow is available on the following lifecycles: Draft to Approved for Use, Draft to Current, and Draft to Final.
- Draft to Approved for Use Lifecycle Usability Enhancements
This feature provides an updated look and feel for documents using the Draft to Approved for Use lifecycle.
Monitor & External User Access Management
These enhancements improve the user administration experience while using the Access and Permissions section of the Monitors and External Users tab:
-
Site List
For multi-site research organizations, only sites to which the monitor or external user currently has access are displayed in the section. -
+Add Site Access
For multi-site research organizations, the +Add Site Access button can be used to add the monitor or external user to an additional site.
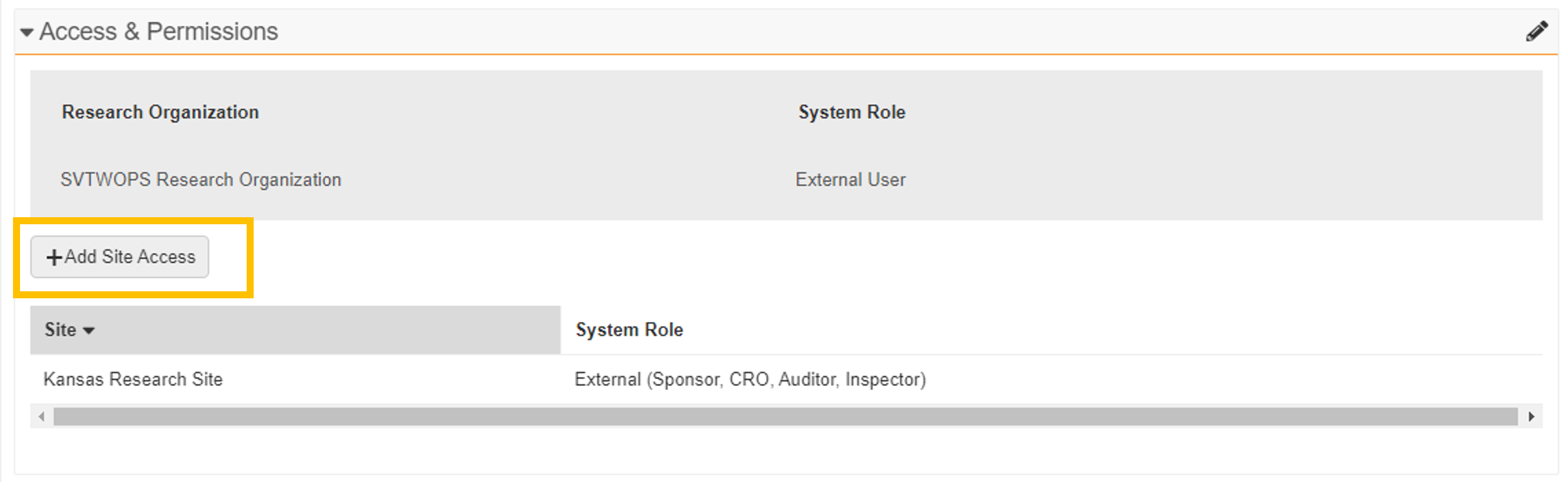
- Remove Access
The Edit User Permission button has been replaced with a pencil icon. When selected, the pencil icon puts the section into Edit mode. While in Edit mode, a Remove Access action is available for any of the listed sites. This action also changes the user’s study assignments to Inactive. When all site access has been removed, the user record automatically changes to Inactive.
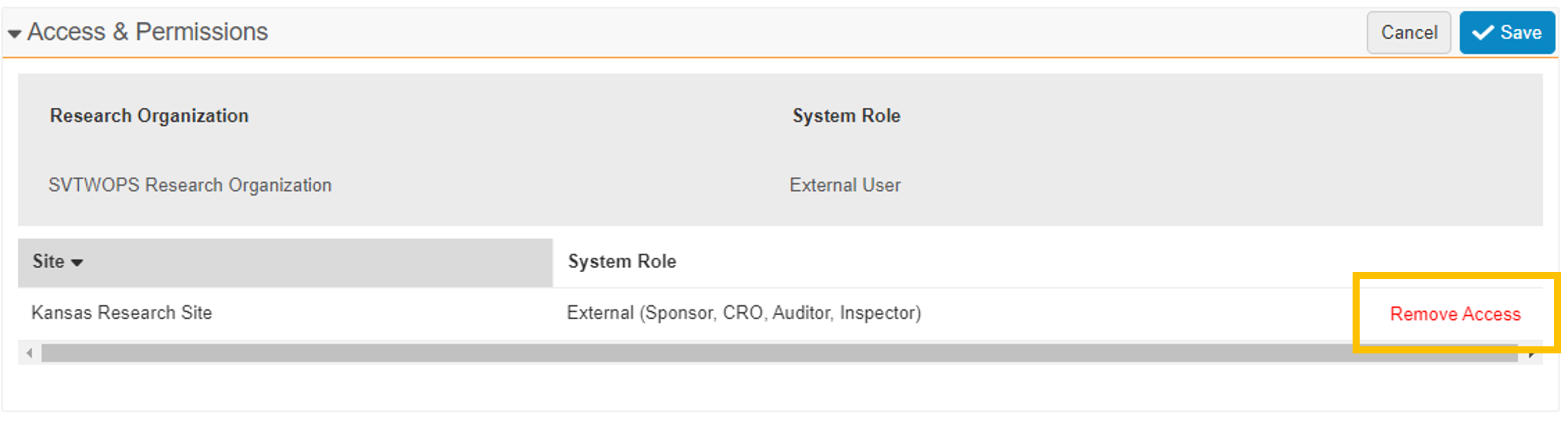
- Automated Study Assignment Status
The Monitor/External User study assignment status is automatically updated to Active when you add a Monitor/External User to a new study (either on the Studies tab or the Monitors and External Users tab), unless you provide a future Scheduled Start Date. Previously, such new study assignments began in the Proposed status. The Monitor/External User study assignment status is automatically updated to Inactive when site access is removed (when a user selects Remove Access).
Attachments Available on all Document Types
Attachments provide a simple way to upload and associate related files with existing documents (for example, certificates of translation for participant-facing materials). With this change, the Document Types spreadsheet will no longer include a column for identifying document types that allow attachments.
Digital Delegation: Flexible Workflows and Start/End Dates
This feature includes multiple changes that add flexibility to the Digital Delegation set-up process.
- Start and End Date Fields
- Various Start and End date fields used with Digital Delegation have been re-labeled for clarity.
- Staff member Start and End Date fields used with Digital Delegation can be edited manually.
- PI Approval Flexibility
PI Approval can be initiated at any point, if at least one staff member has completed acceptance.
For more information, see Sending Delegations for Acceptance and Approval.
Grant Veeva Support Access to SiteVault
This feature enables you to grant Veeva Support access to your SiteVault account. When you need assistance from Veeva Support, it can be useful for them to see your SiteVault from your perspective; this Profile tool is used to set a timeframe for Support to access your SiteVault.
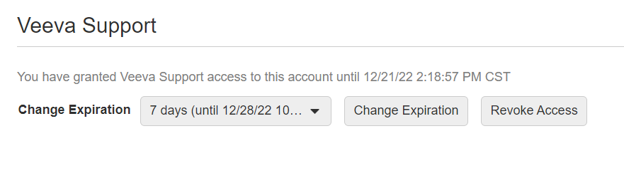
For more information, see Managing Your Profile.
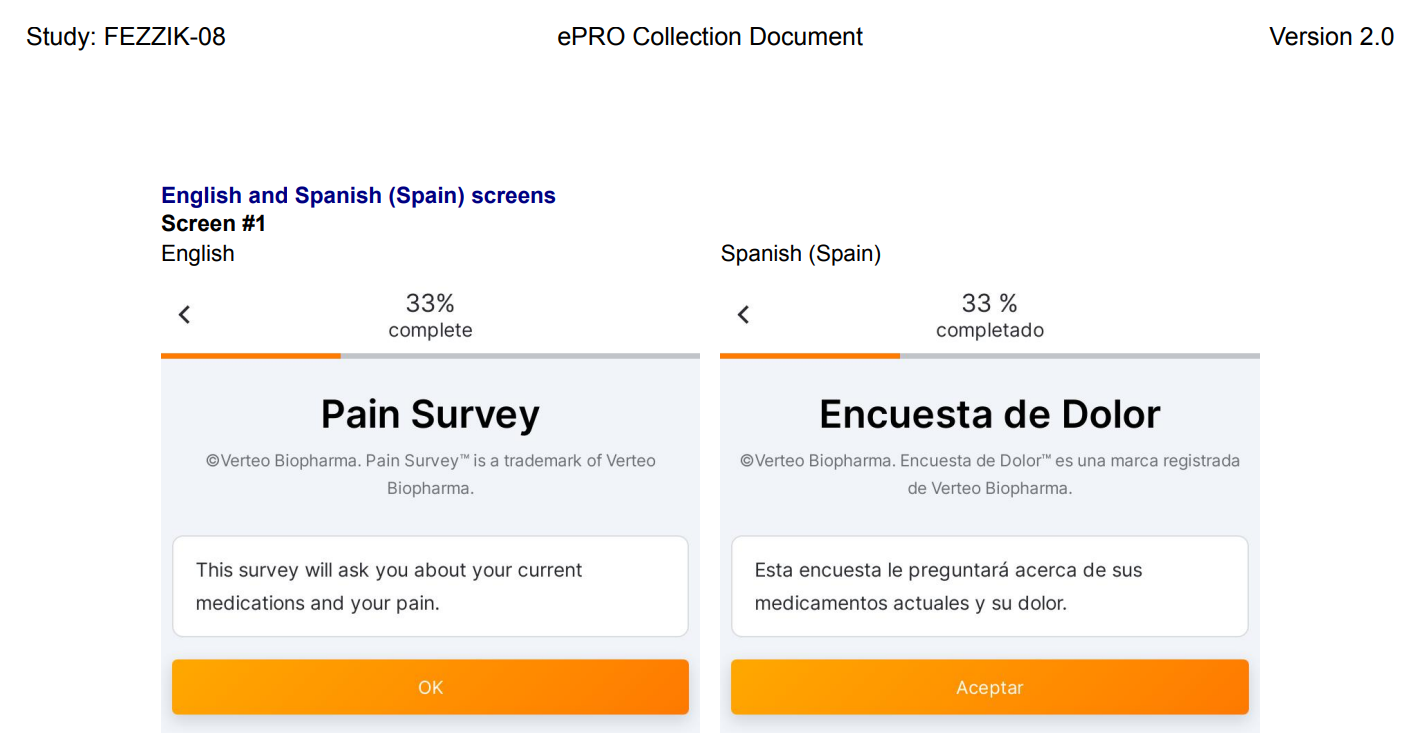
Language Label Change: Spanish to Spanish (Spain)
This feature does not change any technical functionality, but adds clarity to language labeling. The Spanish language options for patients and patient-related materials are Spanish (Spain) and Spanish (Mexico). All patients and related patient-related material that are currently assigned the Spanish language will be assigned Spanish (Spain).
- Previous Spanish Labels
- Spanish
- Spanish (Mexico)
- New Spanish Labels
- Spanish (Spain)
- Spanish (Mexico)
MyVeeva Patient Password Reset
With this feature, Site Admins and Site Staff working in eReg can initiate a password reset on behalf of a MyVeeva user.
Participant Enhancements (eConsent and ePRO)
These enhancements improve the user experience when managing Participants in eReg:
-
Participant Visit Scheduling
Note: This feature requires the patient to have a MyVeeva account. This feature enables you to invite participants to upcoming visits using calendar applications such as Outlook and Google Calendar directly from SiteVault. When you include the MyVeeva calendar alias in your invitation, the event will be available to patients in MyVeeva for Patients. -
Prevent Further Changes to a Participant’s Patient ID
This feature prevents you from changing a study participant’s Patient ID after the participant is invited to use MyVeeva for Patients. -
Participant ID Harmonization
On a Veeva EDC-enabled Connected Study, when you create a new participant record or edit an existing Participant ID, an automated check determines if a Participant ID is present in Veeva’s EDC. Study Connect users can view the participant’s EDC status in the EDC Status field, on the Participant record, along with a link to the participant’s casebook in EDC.
eConsent Enhancements
These enhancements improve the user experience within the SiteVault eConsent functionality:
- Automatically Close eConsent Editor Session and Refresh SiteVault Session Upon Checking In eConsent Form to Vault
This feature makes the following enhancements to the eConsent form check in process (from eConsent editor to Vault):- When you check in an eConsent form, the editor window automatically closes.
- When you check in an eConsent form, the page that launched the editor (either the eReg Document Viewer or the Study Connect Blank Forms) automatically refreshes with the eConsent form’s new content.
- Approve and Send Site-Specific eConsent Form Template to Sponsor/CRO in SiteVault eReg
This feature allows you to approve and send a connected eConsent form to the study’s sponsor/CRO in SiteVault eReg after making site-specific edits in the eConsent editor.
- Create New Draft of Connected eConsent Template in Study Connect
This feature allows you to create a new draft of a blank, connected eConsent form template by:- Creating a copy of the sponsor template in Study Connect
- Creating a copy of the most current version in Study Connect
- Uploading a file from your computer in Study Connect
SiteVault Study Connect
Grid Column Ordering and Name Column Locking
The following enhancements improve the user experience within the Study Connect main grids (tables):
-
Fixed First Columns
When you scroll to the right, the first column in the grid remains fixed in place. -
Flexible Column Order
You can reorder the grid columns on certain pages by dragging the column header.
Notification Bell for Document Exchange, Safety Distribution, and eConsent
With this feature, you can view notifications from any study-specific Study Connect tab. When the Notifications Bell is selected, a panel of notifications is displayed on the right, sorted to notifications related to the feature currently selected in the Study Connect main menu. Filters can then be removed or added as necessary.
Document Exchange Enhancements
The following enhancements improve the user experience within the Document Exchange functionality:
- Sponsor/CRO Document Name
On a received document, you can view the name of the document as it appeared in the sponsor/CRO Vault. This field can be viewed in the All Documents and Received Documents tabs of Document Exchange.

-
Sponsor/CRO Document Number
On a received document, you can view the number of the document as it appeared in the sponsor/CRO Vault. This field can be viewed in the All Documents and Received Documents tabs of Document Exchange. -
Document Version on Send
When sending an eReg document, the version number of the document is displayed in the document name. -
Link to eReg Document
With this feature, you can now quickly access a Study Connect’s associated eReg document copy. An eReg document icon is available in the Document Actions, All Documents, Sent Documents, and Received Documents tabs of Document Exchange.
ePRO Collection Document Requiring Action Displays Visual Indicators in Study Connect
With this feature, you are now notified when an ePRO collection document requires attention. A blue dot indicator on the Studies tab and an orange Action Needed icon on the ePRO tab are displayed when an ePRO collection document requires action from a site user.
Approve and Send Site-Specific eConsent Form Template to Sponsor/CRO in Study Connect
This feature allows an eConsent form template that has been edited in the eConsent editor to be approved and sent to the connected study’s sponsor/CRO in Study Connect.
Participant Profile Enhancements
The following enhancements improve the user experience within the Participant tab:
- Participant ID Harmonization
On a Veeva EDC-enabled Connected Study, when you create a new participant record or edit an existing Participant ID, SiteVault checks if a Participant ID is present in Veeva’s EDC. The participant’s Veeva EDC Casebook status is displayed in the Veeva EDC Casebook field.- Veeva EDC Casebook Field: This new field provides the status of the automated check. The following statuses are available:
- Searching: The system is still actively conducting the automated check.
- Open Casebook: A casebook matching the Participant ID was found and those with the required permissions can click the status to link directly to the casebook in the sponsor’s Veeva EDC system.
- Not Found: The Participant ID was not located in Veeva’s EDC.
- Veeva EDC Casebook Field: This new field provides the status of the automated check. The following statuses are available:
-
Participant Visit Scheduling
Note: This feature requires the patient to have a MyVeeva account. This feature enables you to invite participants to upcoming visits using calendar applications such as Outlook and Google Calendar directly from SiteVault. When you include the MyVeeva calendar alias in your invitation, the event will be available to patients in MyVeeva for Patients. -
Countersign eConsent Form From Participant Profile in Study Connect
This feature allows you to countersign an eConsent form from the consenting participant’s profile page. -
Cancel Signed eConsent Forms From Participant Profile in Study Connect
This feature allows you to cancel a signed eConsent form from the consenting participant’s profile page. -
Description Column for Signed Forms on Participant Profile
This feature adds a Description column to the Signed Forms section on the participant’s profile page. -
Participant Medical Record Number Field
This feature adds a Medical Record Number field to the participant’s profile page. - Pagination Added for Ease of Use
- Form Responses
- Signed Forms
- Participant Events
Veeva eConsent
eConsent Form Templates Allow Site Wet Ink Signatures
This feature allows sponsor and site users to add a site signature block to an eConsent form template to support study countries that require wet ink signatures on consent forms and participants who choose not to sign electronically in MyVeeva for Patients. Site users can now print a study’s eConsent form template and upload a scanned, signed copy to SiteVault in these situations.
For more information, see the following help topics:
- Content Block Settings in Clinical Vault Help
- Consenting Participants In Wet Ink in SiteVault eReg Help
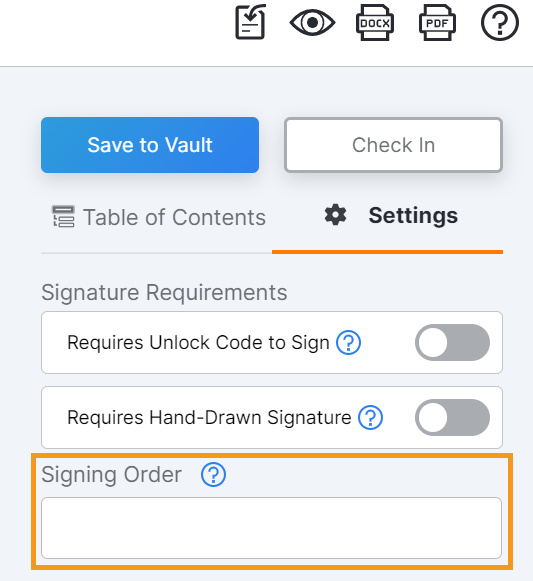
Signing Order
This feature allows sponsor and site users to require MyVeeva users to complete eConsent forms in a specific order. Users can apply numeric signing order values to forms in the eConsent editor, and MyVeeva users must complete the forms in sequential order (for example, 1, 1.1 , then 2).
For more information, see the following help topics:
- Requiring a Signing Order in Clinical Vault Help
- Consent Forms in MyVeeva for Patients Help
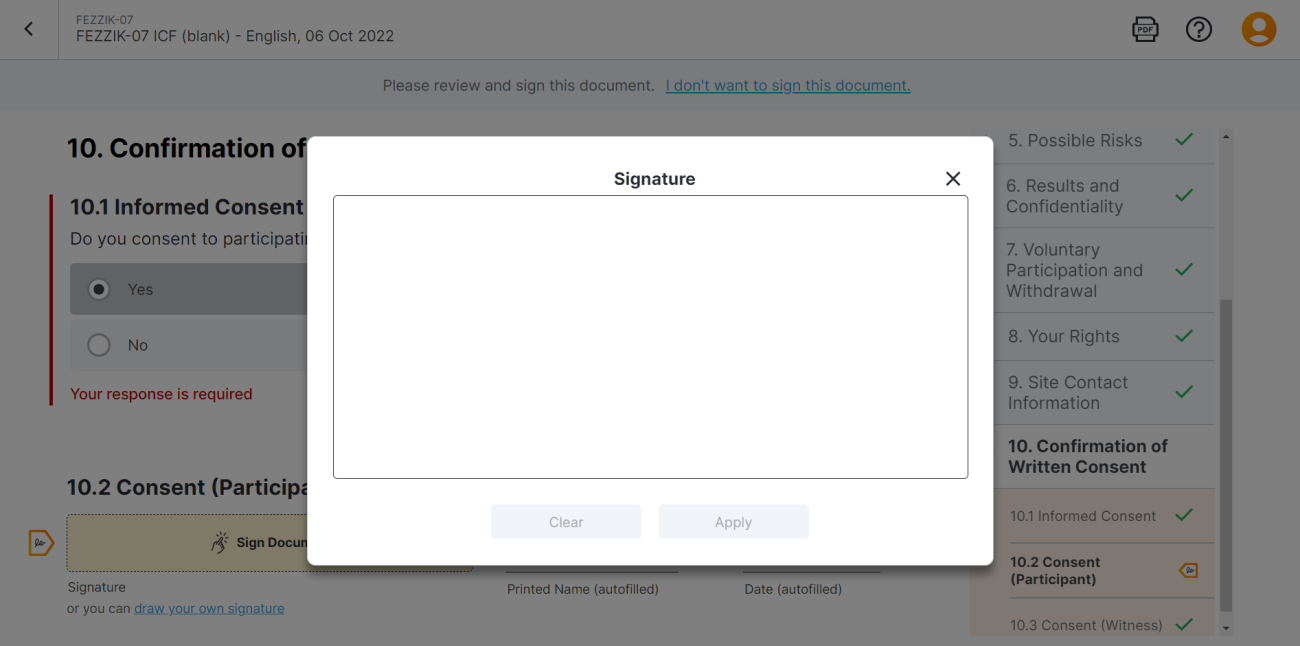
Web Hand-Drawn Signature Modal
This feature improves the hand-drawn eConsent signature experience for MyVeeva users in the web app by aligning it with the experience in the Android and iOS apps.
For more information, see Consent Forms in MyVeeva for Patients Help.
Veeva ePRO
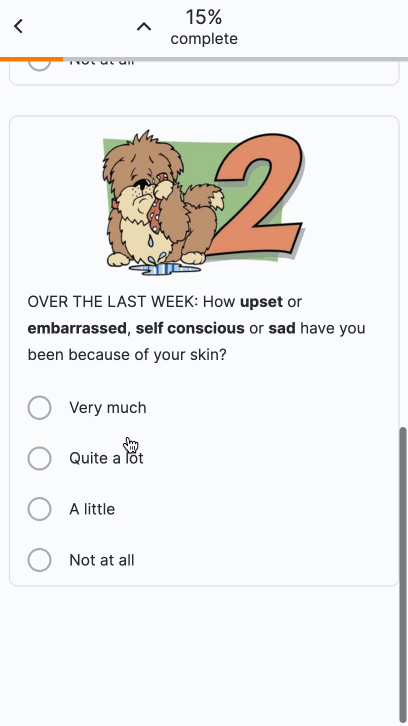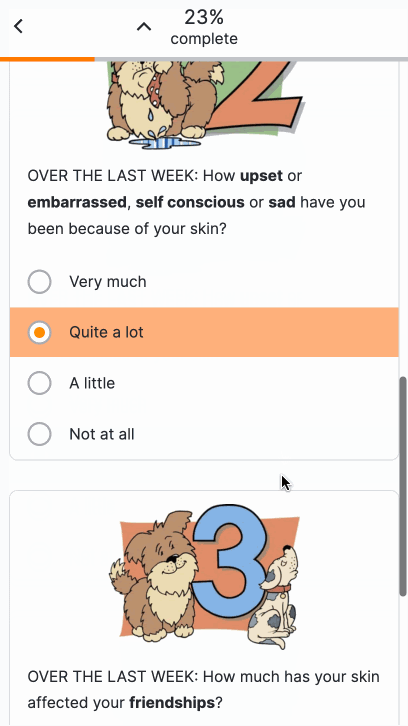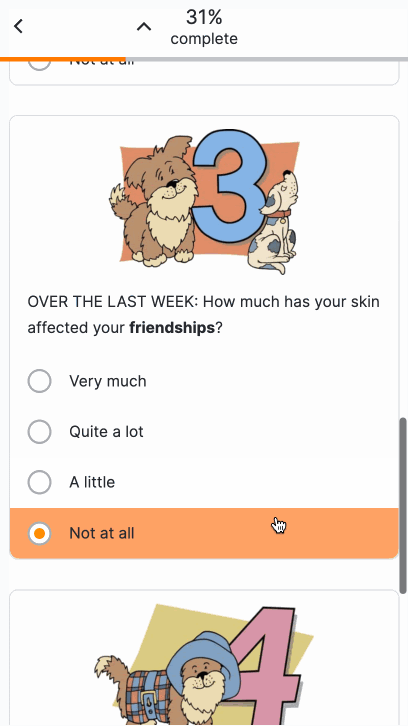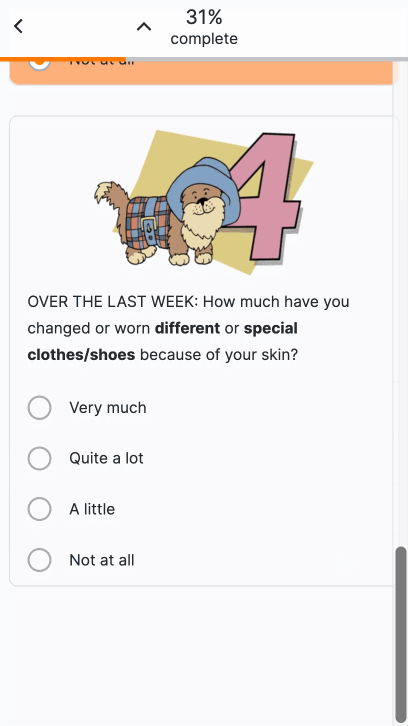
Images in Surveys
This feature allows sponsor users to upload images for use in surveys. After uploading an image, users can add images to appear above license text, above question or instruction headings, above numeric rating scales, or as answers to single- or multiple-choice questions. Users can enter descriptive image text for MyVeeva users to read with a screen reader.
For more information, see the following help topics:
- Uploading Images to Surveys in Clinical Vault Help
- Surveys in MyVeeva for Patients Help
Text Entry Survey Component
This feature allows sponsor users to configure a survey that contains a text entry question for MyVeeva users to complete. Sponsor users must configure a maximum character limit for responses.
For more information, see the following help topics:
- Configuring a Text Entry Question Type in Clinical Vault Help
- Surveys in MyVeeva for Patients Help
Multiple-Choice Dropdown Survey Component
This feature allows sponsor users to configure a survey that contains a multiple-choice question with response options displayed in a drop-down menu for MyVeeva users to complete.
For more information, see the following help topics:
- Configuring a Multiple-Choice Question Type in Clinical Vault Help
- Surveys in MyVeeva for Patients Help
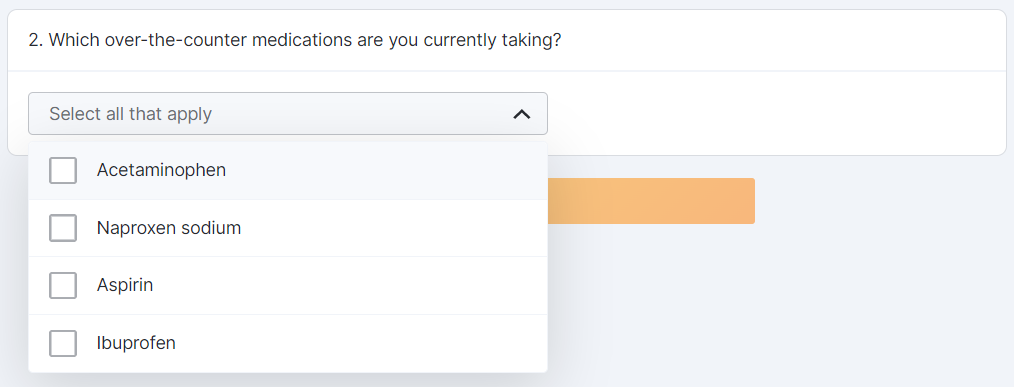
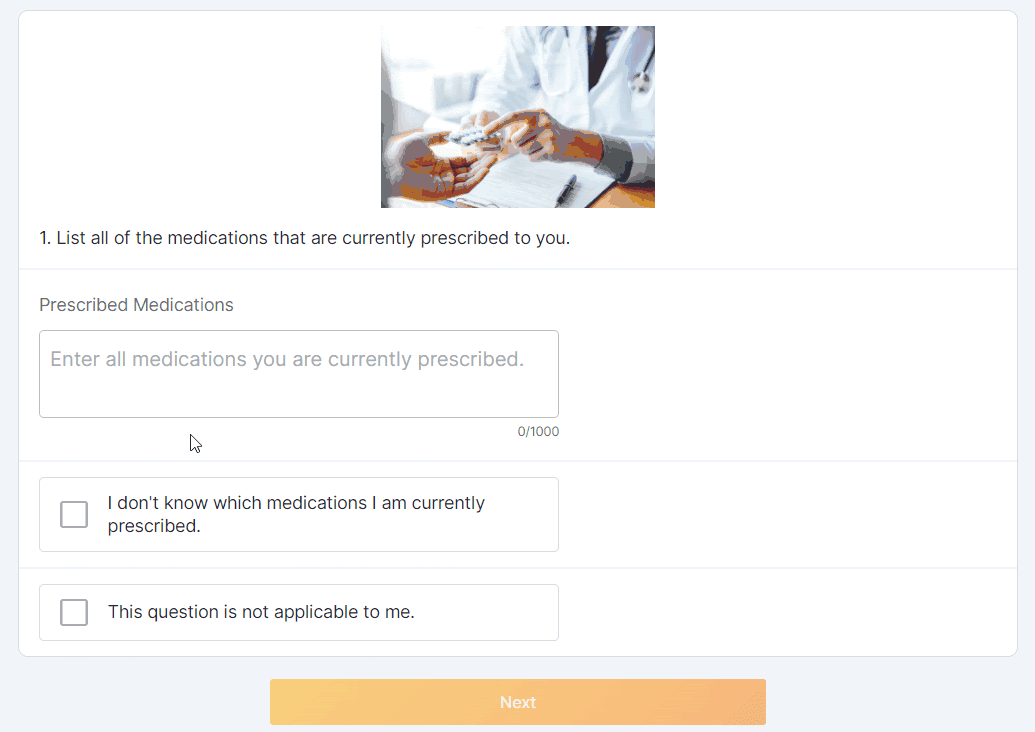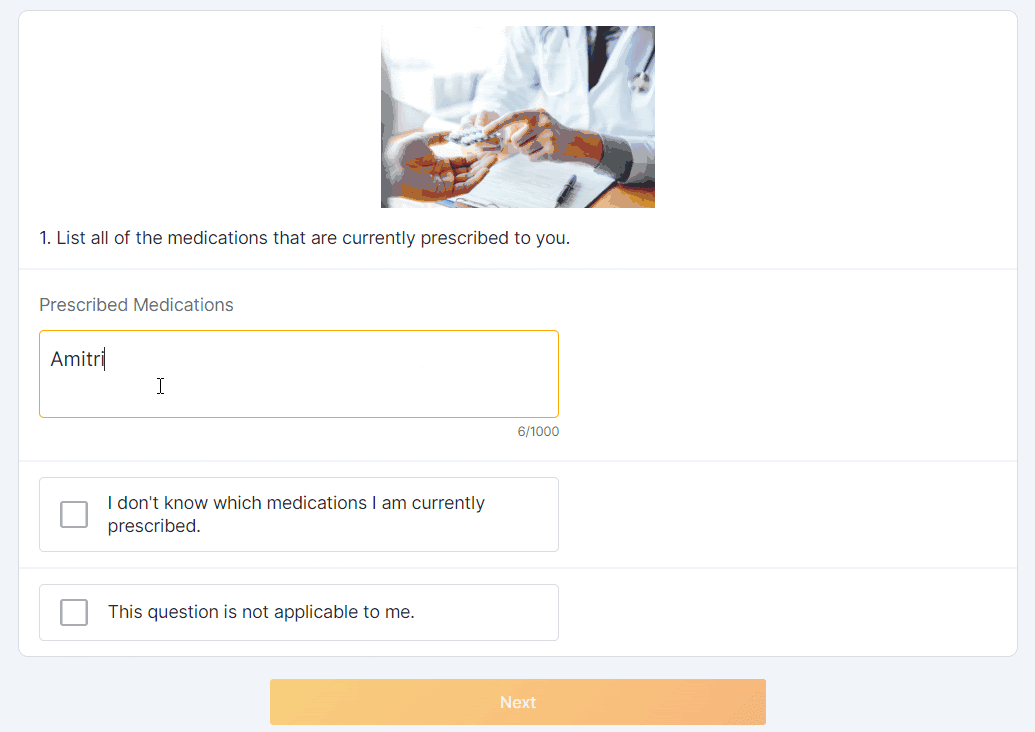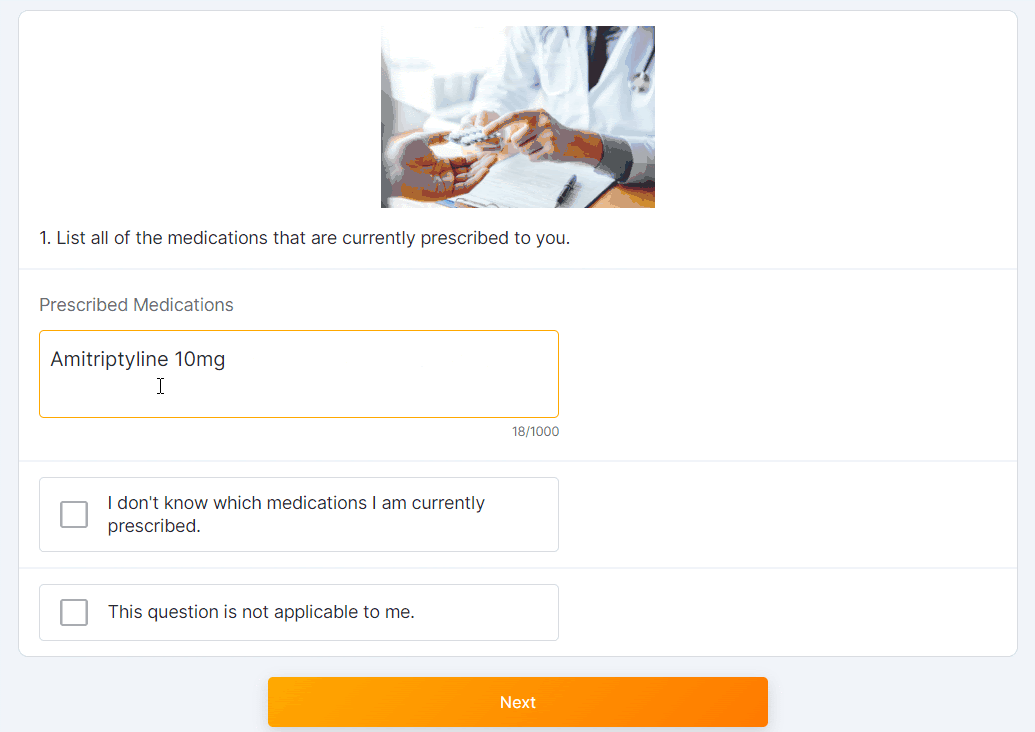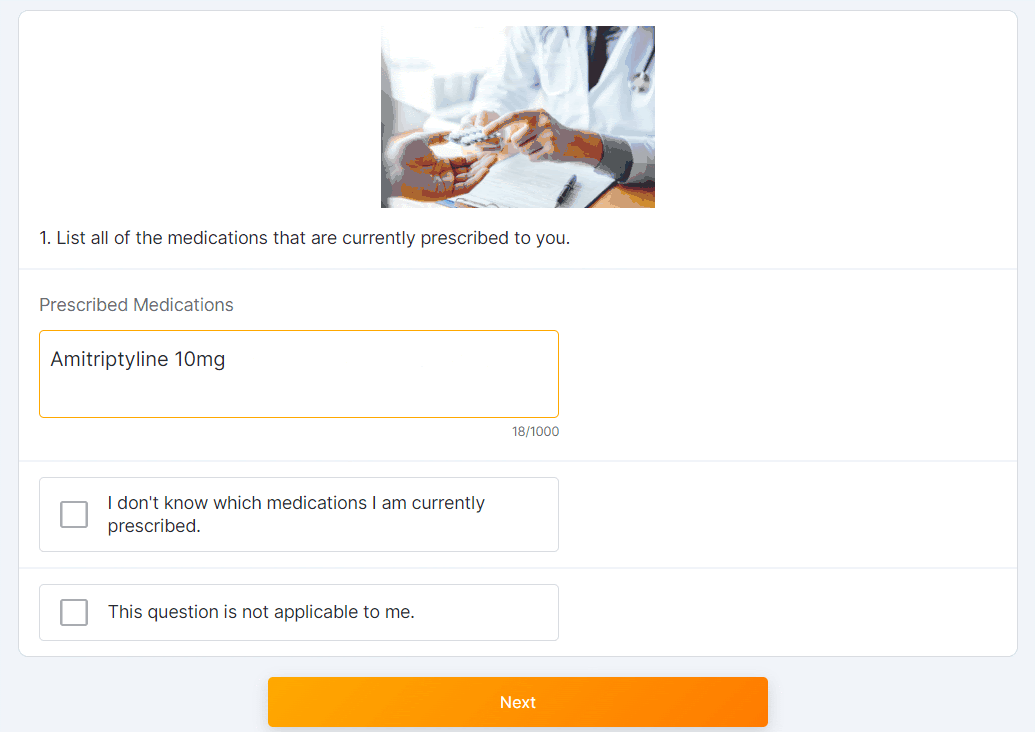
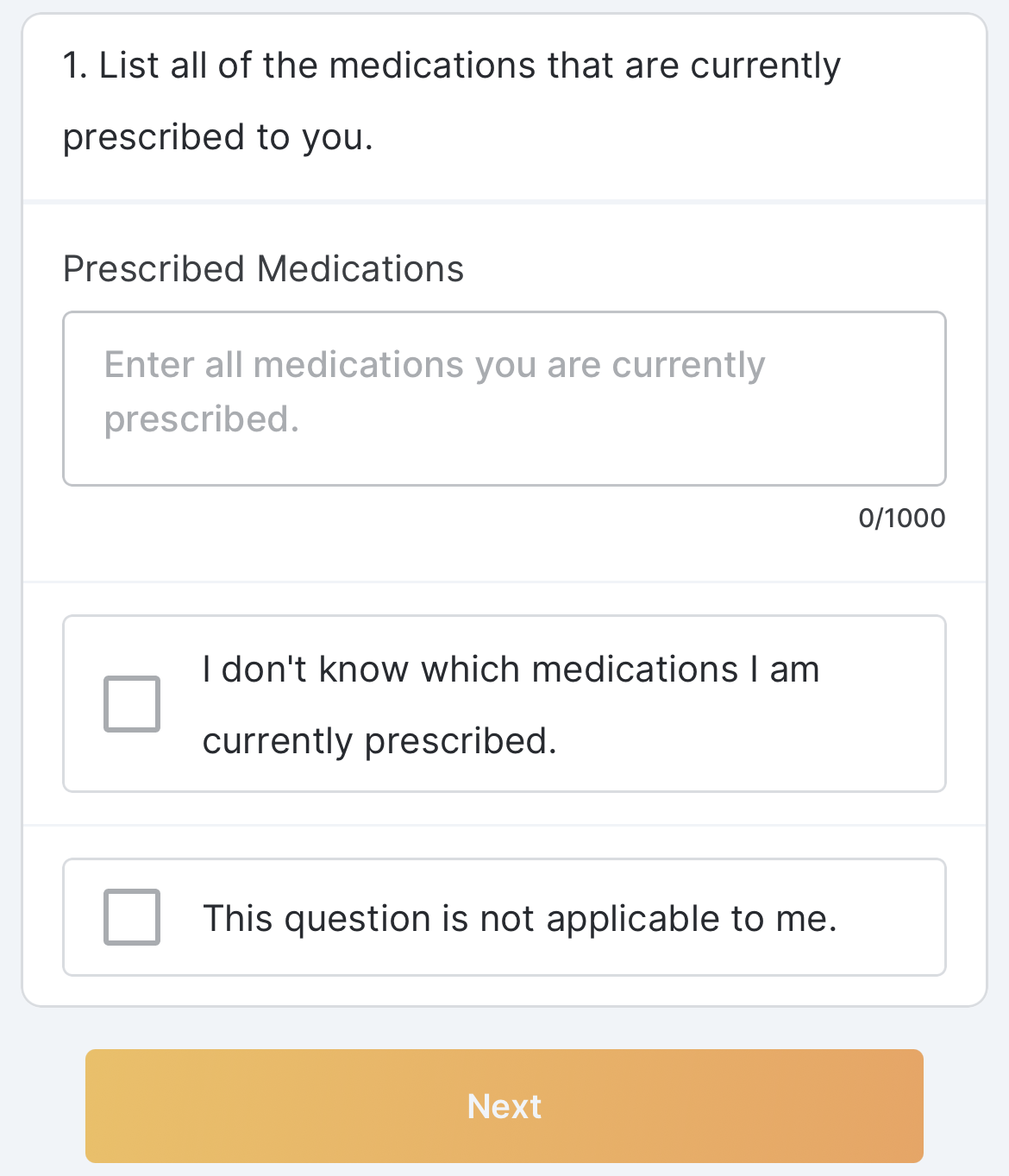
Optional Question Survey Component
This feature allows sponsor users to configure a survey with optional questions. Sponsor users can configure one or multiple optional responses for a survey question (for example, “This question is not applicable to me”). MyVeeva users can select an optional response to proceed to the next question without submitting a standard response.
For more information, see the following help topics:
- Universal Survey Block Parameters in Clinical Vault Help
- Surveys in MyVeeva for Patients Help
Survey Sequence
This feature allows sponsor users to enter numeric survey sequence values for surveys in an ePRO collection. MyVeeva users that receive surveys with sequence values are required to complete the surveys with lower sequence values before those with higher sequence values.
For more information, see the following help topics:
- Configuring Survey Sequence in Clinical Vault Help
- Surveys in MyVeeva for Patients Help
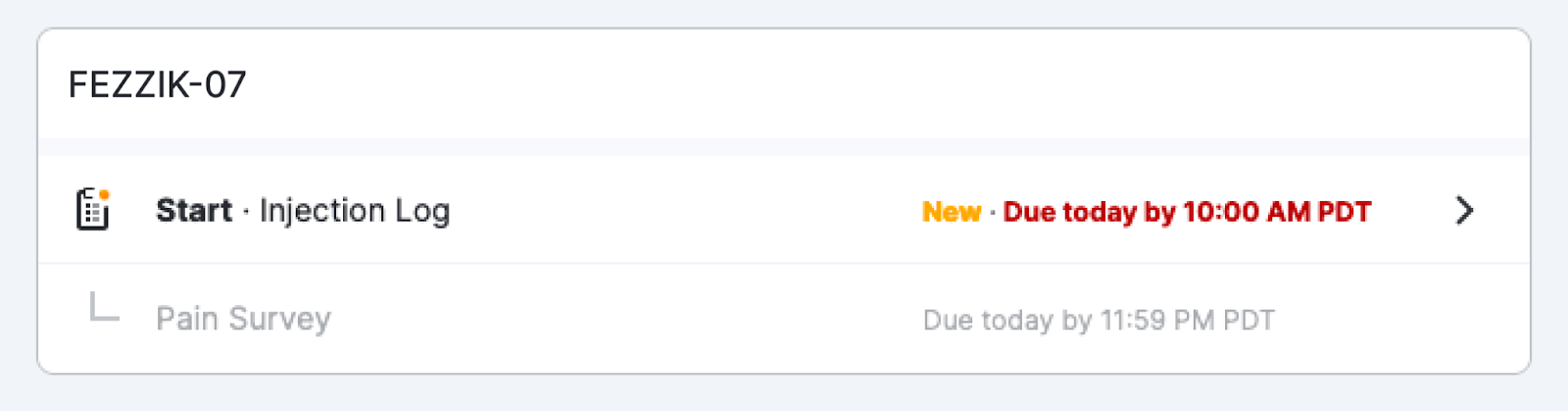
Survey Preview Links Included in ePRO Collection Document
This feature allows sponsor and site users to access survey preview links from within an ePRO collection document. Survey preview links allow users to preview a study’s surveys in a variety of device sizes and study-supported languages.
For more information, see the following help topics:
- Using an ePRO Collection in Clinical Vault Help
- Managing ePRO Collection Documents in Study Connect Help
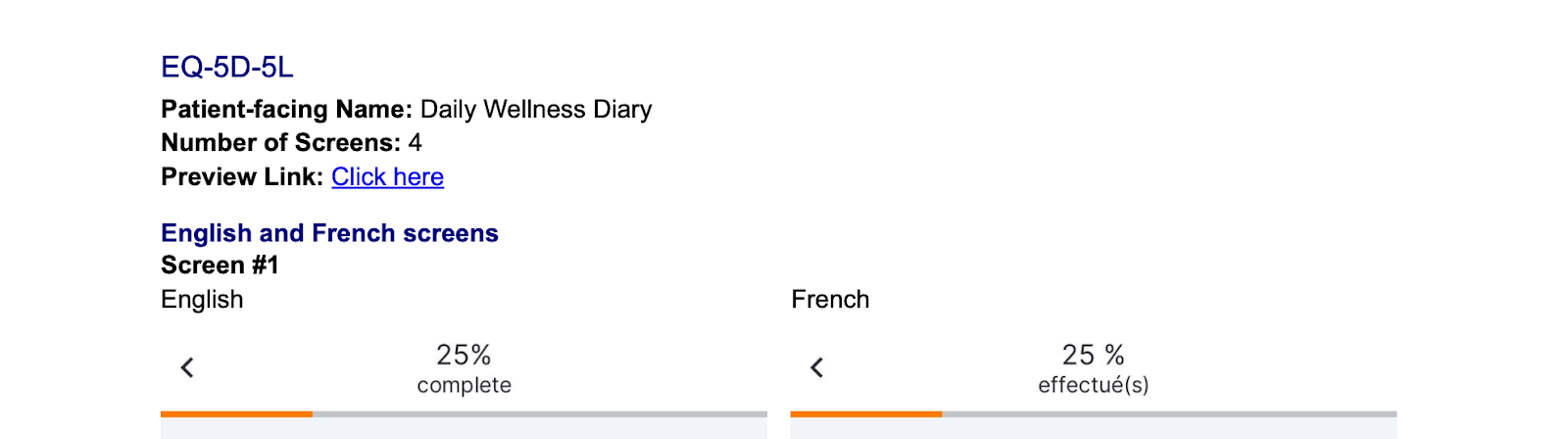
Side-by-Side English and Non-English Screenshots in ePRO Collection Document
This feature enhances ePRO collection documents for sponsor and site users by displaying non-English screenshots next to their corresponding English screenshots. Previously, non-English screenshots were displayed without their English counterparts.
For more information, see the following help topics:
- Using an ePRO Collection in Clinical Vault Help
- Managing ePRO Collection Documents in Study Connect Help
Sites Only Receive New ePRO Versions That Affect Their Site
This feature allows site users to only receive a new version of ePRO if a sponsor user makes changes affecting a study language that the site supports (such as by adding a new survey or updating translations for a site-supported language).
For more information, see the following help topics:
- Upversioning an ePRO Collection in Clinical Vault Help
- Managing New Collection Document Versions in Study Connect Help
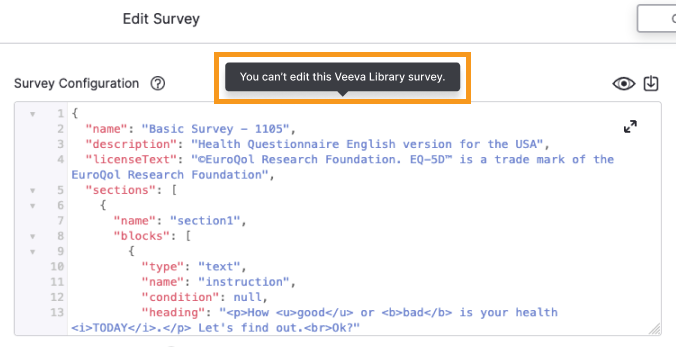
Sponsors Prevented from Editing Licensed Veeva Library Surveys
This feature prevents sponsor users from making edits to Veeva Library surveys that have been reviewed and approved by license holders. Users can continue to edit Veeva Library surveys that haven’t been reviewed and approved by license holders as well as their own sponsor library surveys.
For more information, see Using Survey Libraries in Clinical Vault Help.
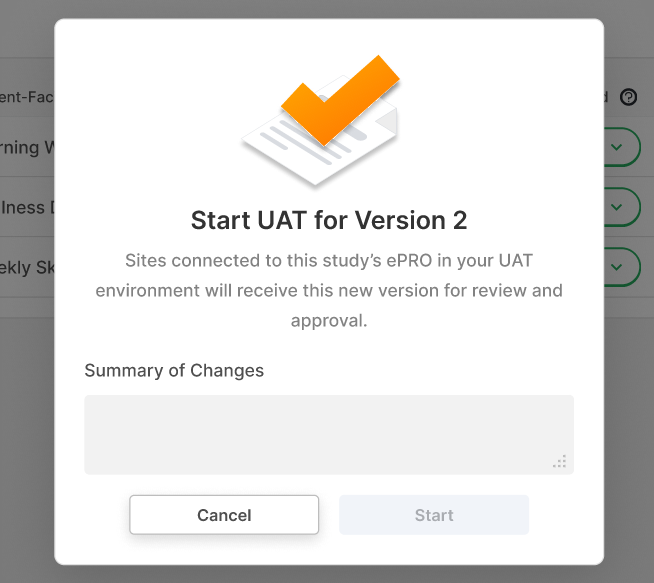
Summary of Changes During ePRO UAT
This feature requires sponsor users to enter a summary of changes while performing UAT on an upversioned ePRO collection. When a user finishes UAT and then approves the ePRO collection, the summary of changes is already provided and can be updated if desired.
For more information, see Performing User Acceptance Testing (UAT) on an ePRO Collection in Clinical Vault Help.
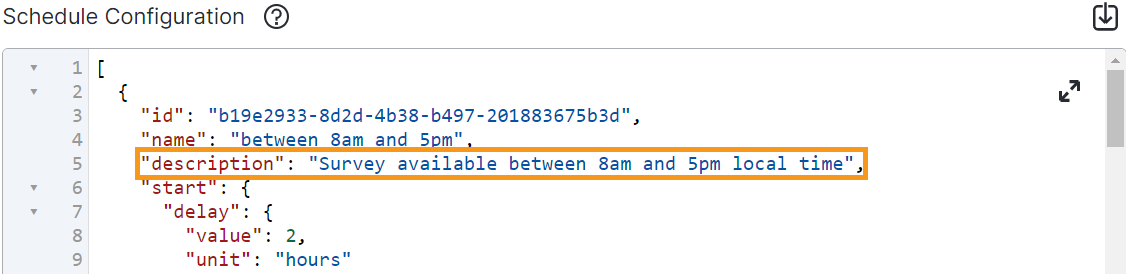
Add Description to ePRO Schedule
This feature allows sponsor users to add a description to an ePRO schedule’s JSON configuration. The description is informational and only displays to users within the ePRO module.
For more information, see Configuring Schedules and Notifications in Clinical Vault Help.
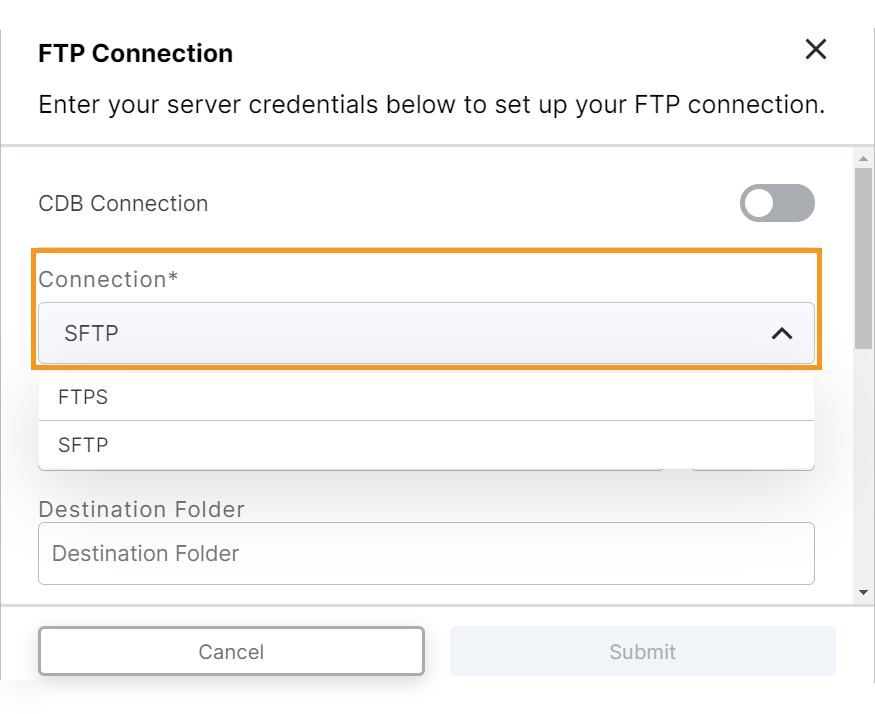
ePRO SFTP Connection
This feature enhances the ePRO FTP connection in the Veeva ePRO module by allowing sponsor users to create connections with SFTP in addition to FTPS, which is already supported. Users can create an SFTP connection by entering their external server credentials, selecting which ePRO data sets are sent, and testing the connection. Once an SFTP connection is established, ePRO data is sent once per day.
For more information, see Creating an FTP Connection to Automatically Transfer ePRO Data in Clinical Vault Help.
Use English for ePRO if Site Hasn’t Selected Supported Languages
This feature defaults a site’s supported languages for a study’s ePRO to English if the site hasn’t selected supported languages for their study, site, or organization.
Additional Sponsor and Site ePRO Data Export Columns
This feature enhances ePRO data exports for sponsor and site users by adding the following columns:
- Sponsor Audit Trail Data now includes a Vault Username column.
- Site Audit Trail Data now includes Participant Global ID and Vault Username columns.
- Site ePRO Responses Data now includes an Answer Unique ID column.
For more information, see the following help topics:
- Exporting Survey, Adherence, and Audit Trail Data in Clinical Vault Help