Note Study Connect features can only be accessed by sites that are invited to a connected study by a sponsor.
About the Release
Important Dates
The release dates below are important for the Digital Trials Platform. See the About the 22R3 Release page in the Vault Release Notes for Vault release dates.
| Date | Event | Description |
|---|---|---|
| October 31 | Pre-Release | The release is deployed to pre-release Vaults and the MyVeeva for Patients pre-release environments. Study Connect is planned to be available December 3, 2022, and will not be included in the pre-release. |
| October 31 - November 4 | Validation Package |
The Digital Trials Platform validation package is available in VeevaDocs on the dates below. The Digital Trials Platform validation package includes validation documents for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO.
|
| December 2 | General Release | The release is deployed to all general release Vault PODs and MyVeeva for Patients general release environments. |
Release Information and Impacts
The release resources and information below are available for the Digital Trials Platform. See the About the 22R3 Release page in the Vault Release Notes for Vault resources and information.
| Resource | Description | Publication History |
|---|---|---|
| Digital Trials Platform 22R3 Release Impact Assessment |
The release impact assessment analyzes the regulatory, configuration, and user impacts of the MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO features in this release. |
Updated November 15 |
| What's New |
The What's New information provides detailed explanations of the new features for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO. |
Updated November 15 |
| Fixed Issues |
The MyVeeva for Patients Fixed Issues list at the bottom of this page documents MyVeeva for Patients issues that affected previous versions or the pre-release and are fixed in this general release. |
Published November 18 |
| Known Issues |
See the following pages for known issues that affect this general release or previous ones and aren't fixed yet: |
|
| Maintenance Releases |
The MyVeeva for Patients Maintenance Releases page lists releases that fix issues affecting the MyVeeva for Patients general release. |
Available with the first maintenance release |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new features across the Digital Trials Platform:
|
See links |
What’s New
MyVeeva for Patients Mobile
Mobile Push Notifications
This feature allows MyVeeva users to receive mobile push notifications if they have the MyVeeva for Patients Android or iOS apps installed on their device. When a user receives a mobile push notification and selects it, they automatically navigate to the MyVeeva for Patients mobile app.
Users must enable or disable mobile push notifications from MyVeeva for Patients in their device’s system settings.
For more information, see Getting Started in MyVeeva for Patients Help.

22R3 Android, iOS, and Web UI Refresh
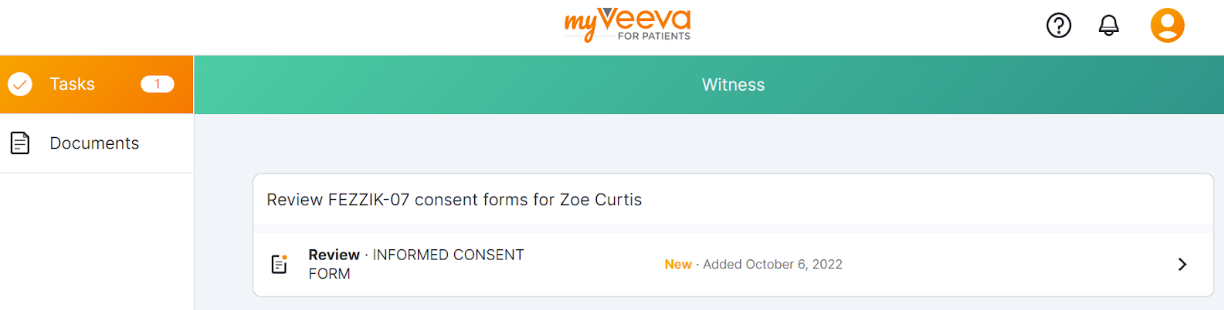
This feature provides MyVeeva users with a refreshed user experience. MyVeeva users using the Android, iOS, or mobile web apps on a smartphone now have a top and bottom navigation system and can select their notification bell, account settings, tasks, and documents from the same screen. MyVeeva users using the mobile web app on a tablet or iPad or the web app on desktop now have a refreshed look to the navigation sidebar. Tasks in a user’s task list have updated designs and statuses.
Users with multiple roles can now change roles by selecting the account icon and selecting Account. When users change to a guardian, proxy, witness, or translator role, a colored banner at the top of the screen indicates which role they’re currently using.
MyVeeva for Patients Platform
Notification Bell Redesign
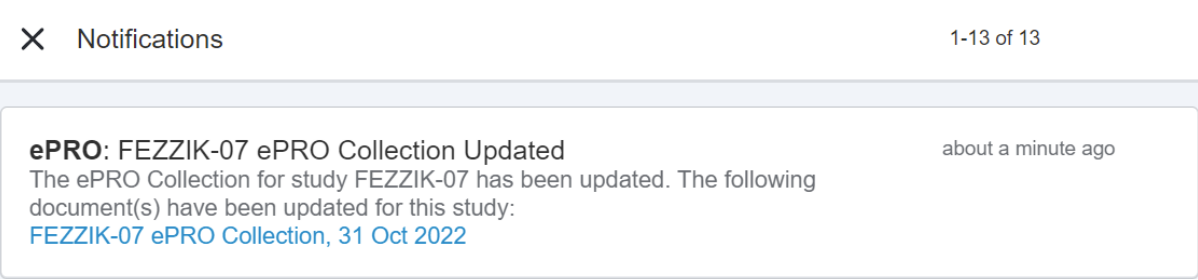
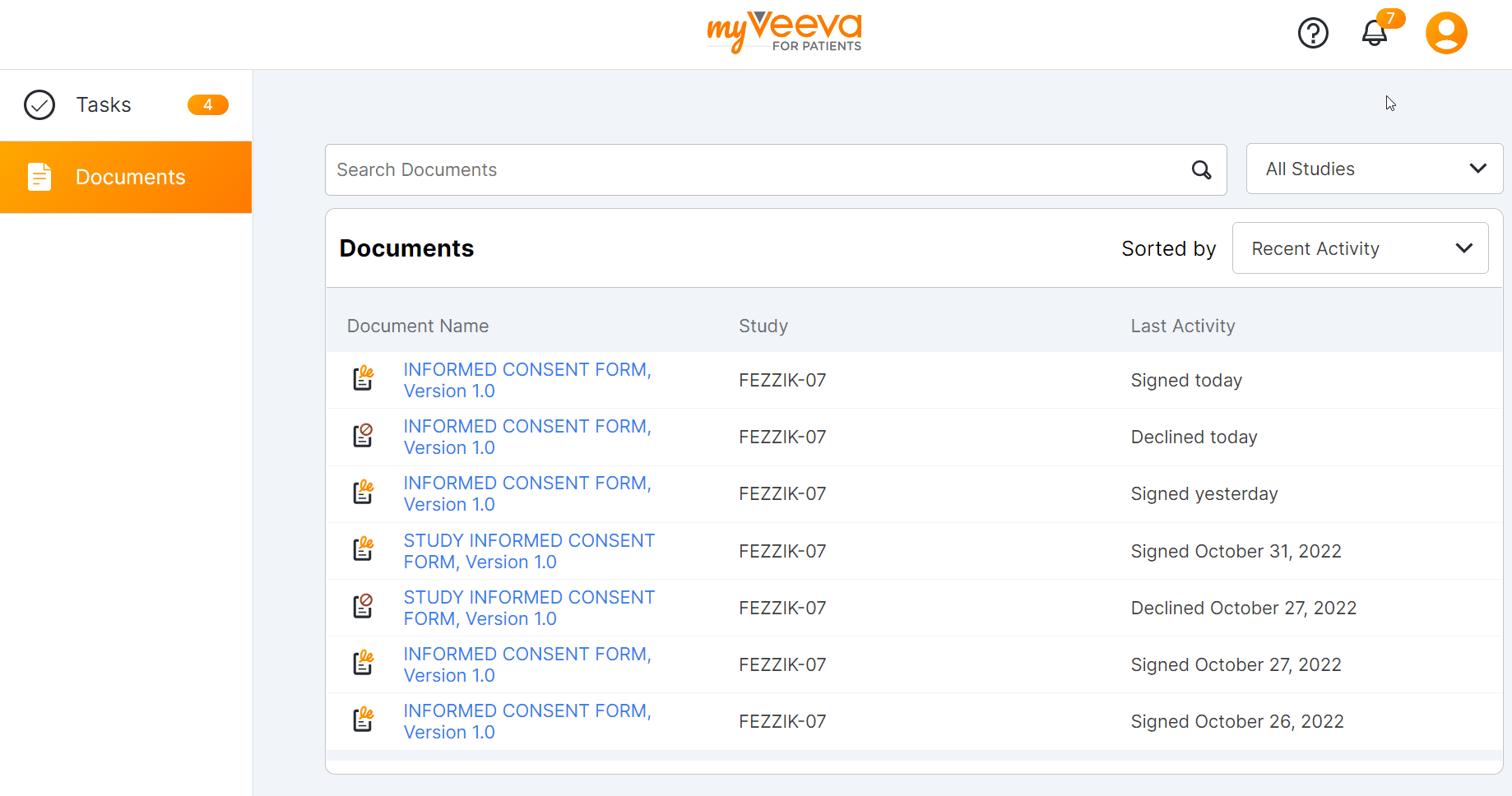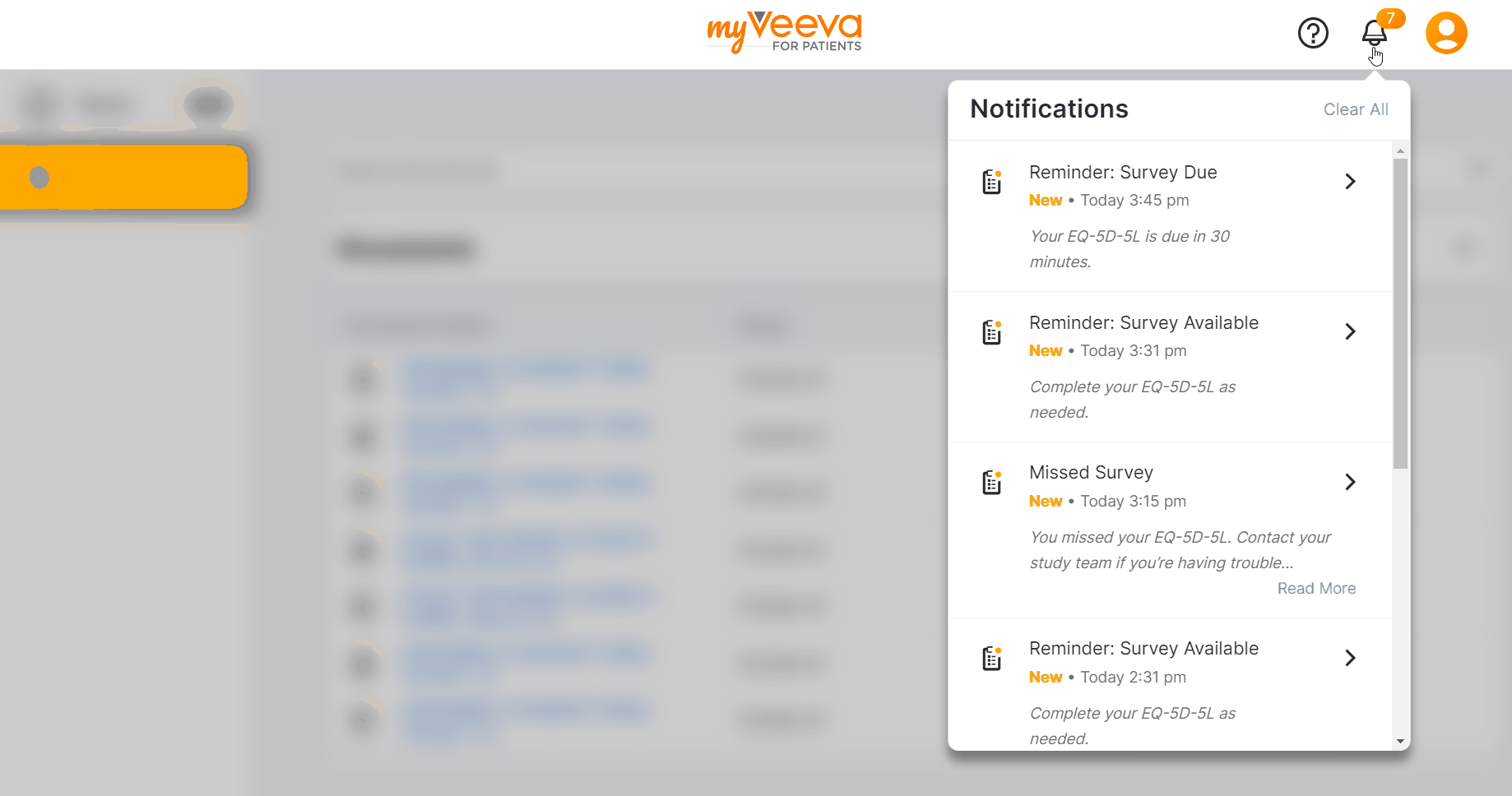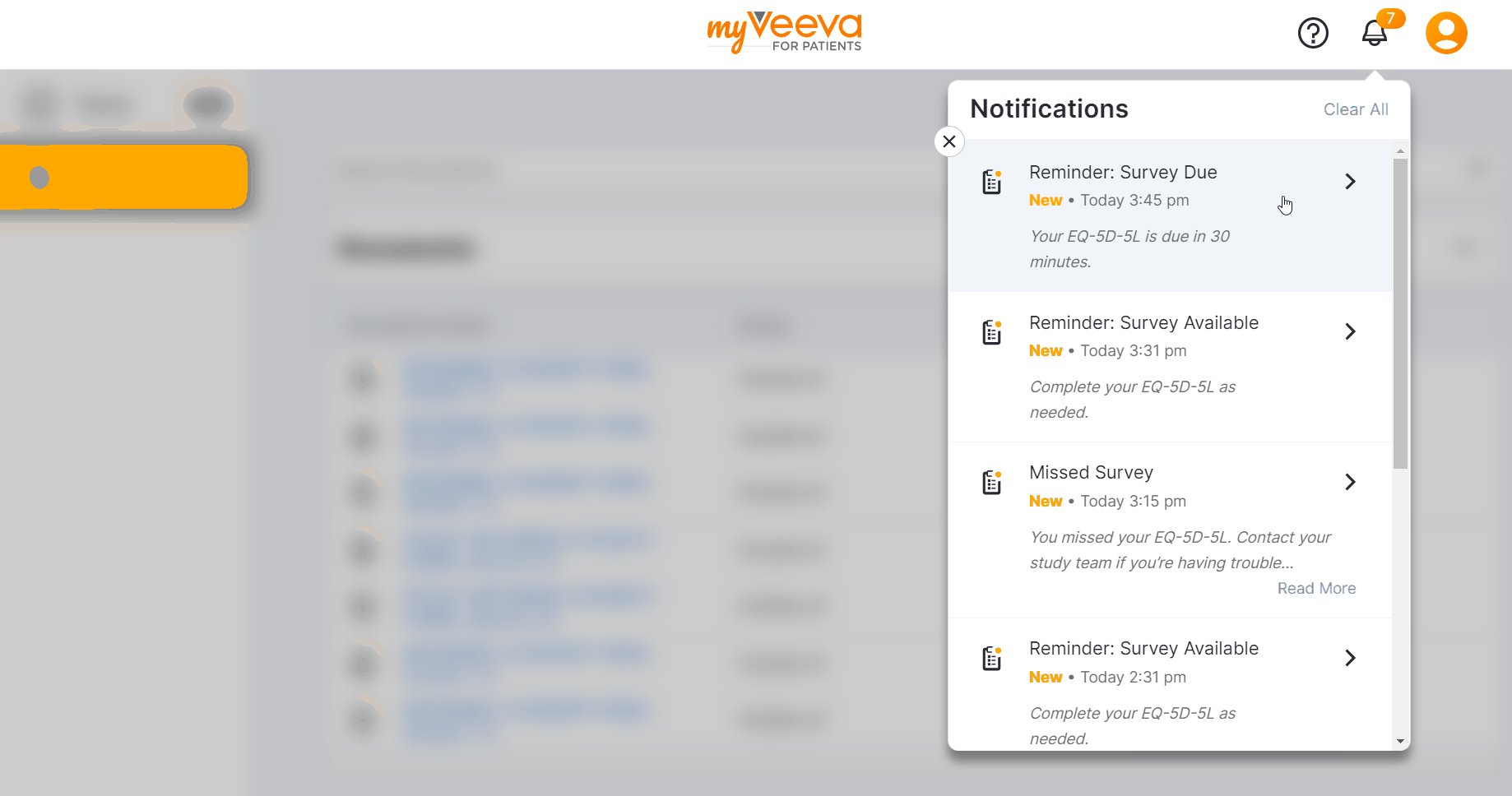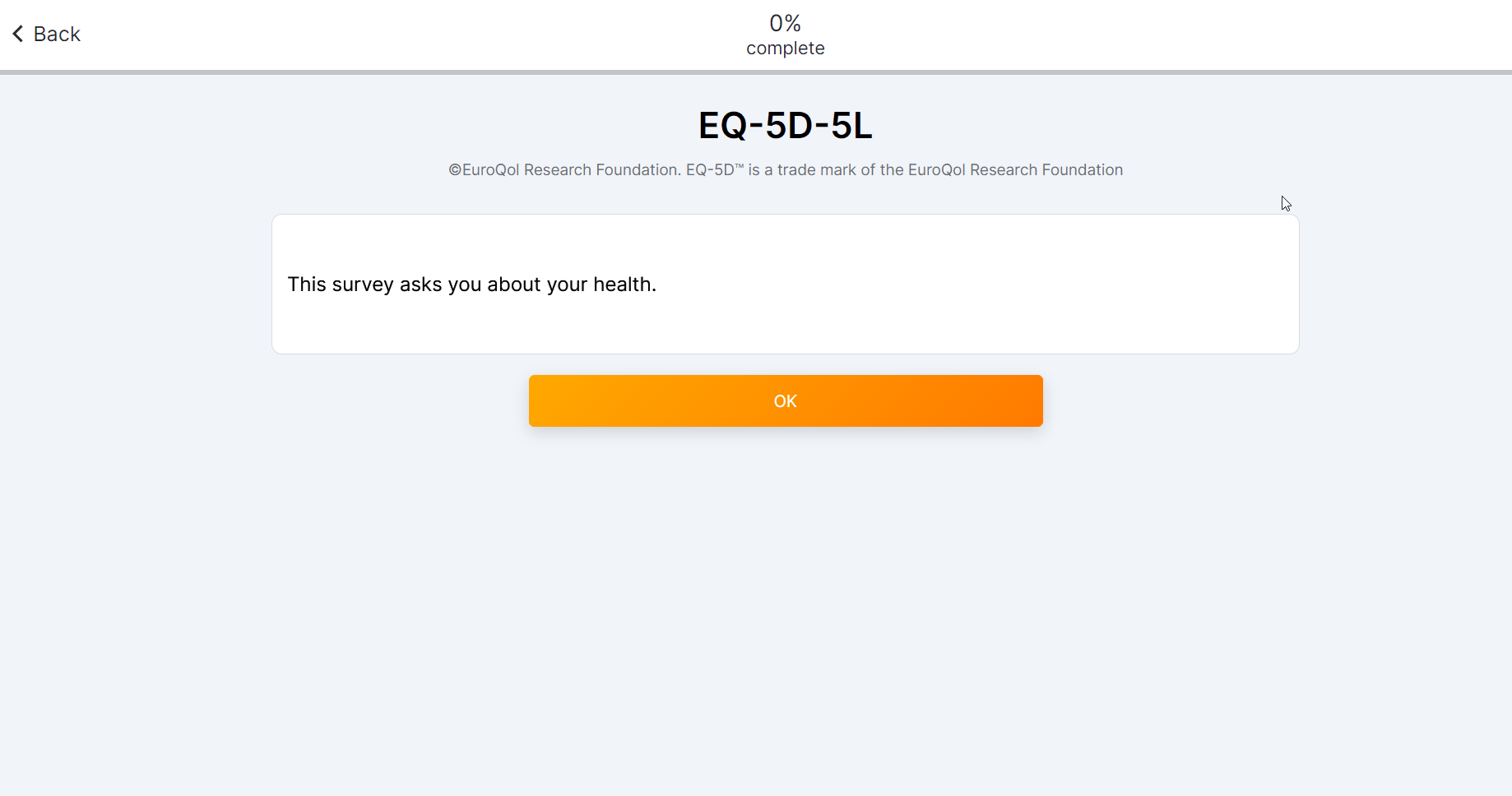
This feature allows MyVeeva users to receive and view their notifications in their notification bell in MyVeeva for Patients. Notifications are sorted by the date and time they’re sent, and an indicator on the bell shows how many unread notifications they have. Users can select a notification from their notification bell and automatically navigate to the task associated with it in the MyVeeva for Patients app.
If a user completes a task related to an unread notification, the notification automatically removes itself from their notification bell. Users can clear notifications from their notification bell one at a time or all at once.
For more information, see Getting Started in MyVeeva for Patients Help.
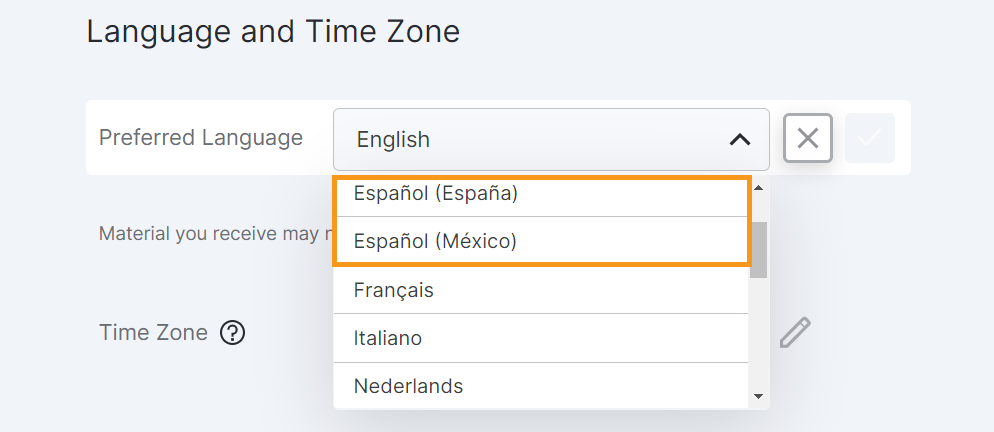
Fallback Languages
This feature allows MyVeeva users to choose their preferred language and locale in their MyVeeva for Patients account settings. If translations aren’t available for the locale-specific language selected by the user, the MyVeeva for Patients Android, iOS, and web apps display messages and labels in the next-best language for their selection.
Users can select their preferred language in their account settings.
For more information, see following help topics:
- Supported Languages in SiteVault eRegulatory Help
- Account Settings in MyVeeva for Patients Help
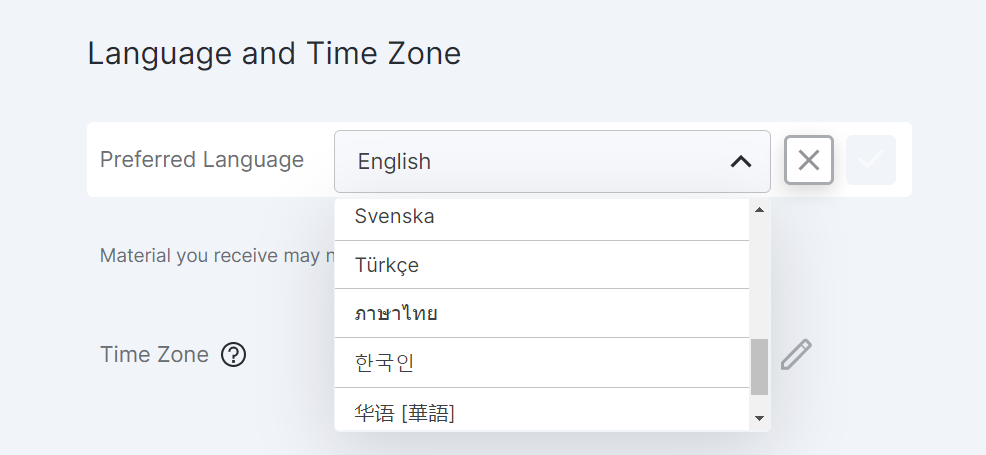
22R3 Translations and Six New Languages
This feature allows MyVeeva users to view application text, emails, text messages, and the terms of use and privacy policy in Norwegian, Singaporean, Spanish (Mexico), Swedish, Thai, and Turkish.
For more information, see Account Settings in MyVeeva for Patients Help.
SiteVault
This release introduces a whole new way to manage your eRegulatory documents and exchange study information with sponsors in SiteVault.
For SiteVault customers currently using SiteVault as an eRegulatory solution, SiteVault is now known as SiteVault eRegulatory and includes a refreshed Study eBinder where study staff and monitors can manage study-related documents all in one place.
For SiteVault customers who exchange documents with sponsors and CROs on connected studies, SiteVault Study Connect offers an enhanced place for sites to securely access and exchange trial information.
SiteVault Study Connect
SiteVault Study Connect - Available December 3, 2022

Study Connect is a new application in SiteVault where site users can complete all of the necessary sponsor-driven activities to effectively conduct a connected study. The Study Connect application is available to sites that are invited to a connected study by a sponsor.
Study Connect includes a tailored user experience for each of the following workflows:
- Document Exchange: Receive and exchange study documents to reduce manual steps and redundant requests.
- Safety Distribution: Receive and acknowledge safety reports in a timely manner.
- eConsent: Provide participants with convenient access to consent forms and key study information.
- ePRO: Capture and manage study participant electronic patient-reported outcomes (ePRO).
With the implementation of Study Connect, you’ll find the following changes:
- Document exchange and safety distribution workflows are now located in Study Connect.
- eConsent and ePRO workflows are now located in Study Connect. Note: Site users on an eConsent- or ePRO-enabled connected study before this release can continue using SiteVault eRegulatory, but shifting to Study Connect will provide a more streamlined experience.
- For SiteVault customers using both applications, you can use the Switch to Study Connect and Switch to eReg buttons in the upper-right corner to navigate between the applications.
- The Regulatory Document Request page is retired as the functionality is now part of Study Connect.
- You now must send person and organization profile documents to a sponsor or CRO using the Send action on the document in Study Connect. With this release, these documents are no longer transferred automatically when a study team assignment or study partner organization are made active on a study.
New Exchangeable Document Types on Connected Studies
This feature adds Participant Adverse Event Log and Protocol Deviations to the list of available document types that can be exchanged with a sponsor or CRO through Study Connect.
This feature also adds support to receive Adverse Event Form templates from sponsors and CROs.
SiteVault eRegulatory
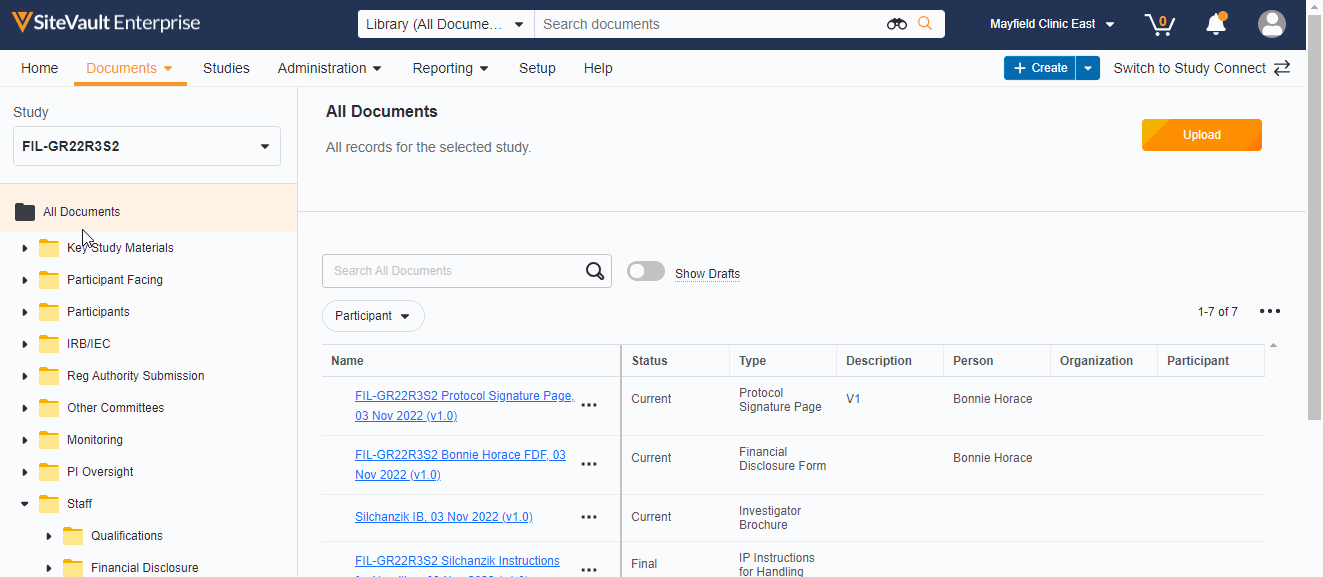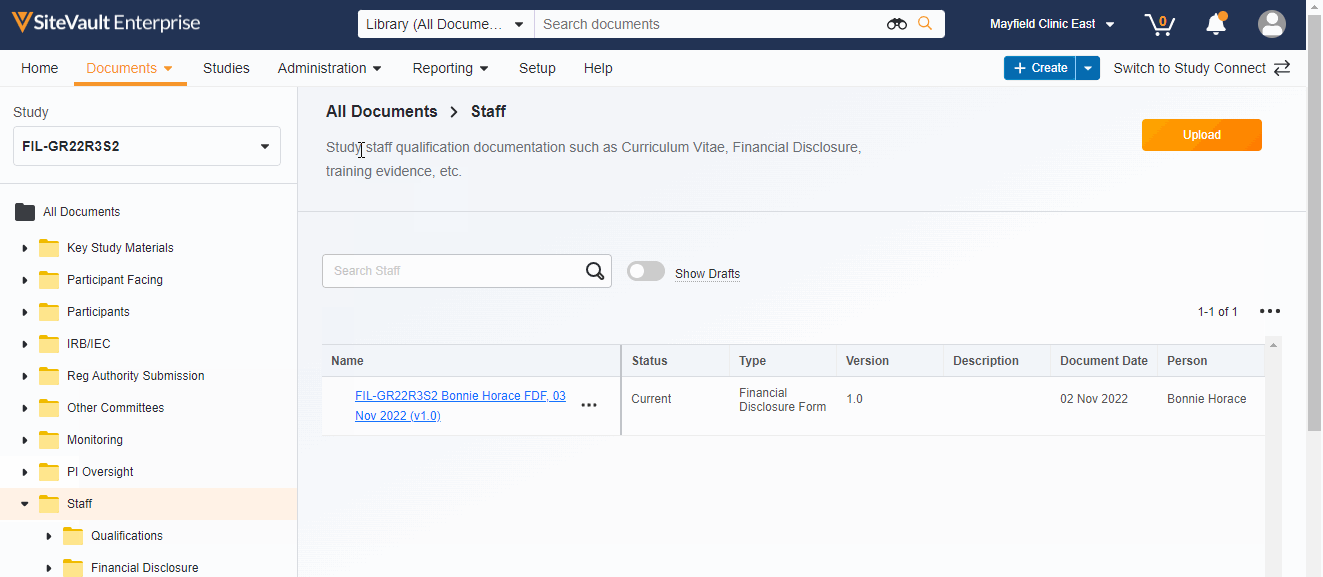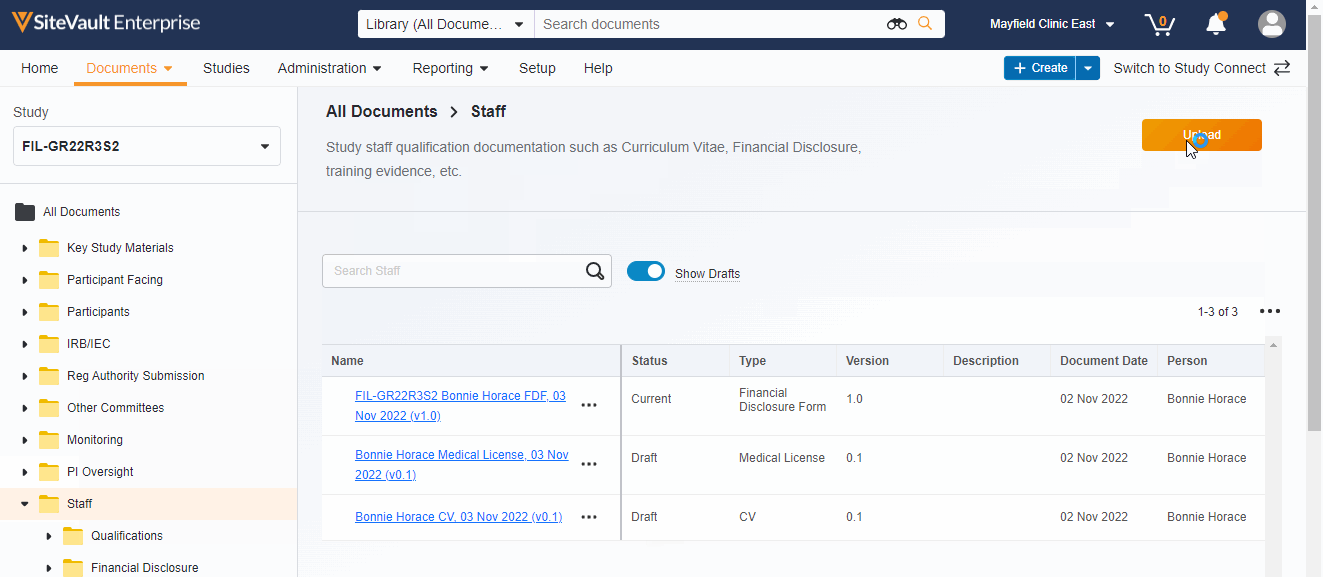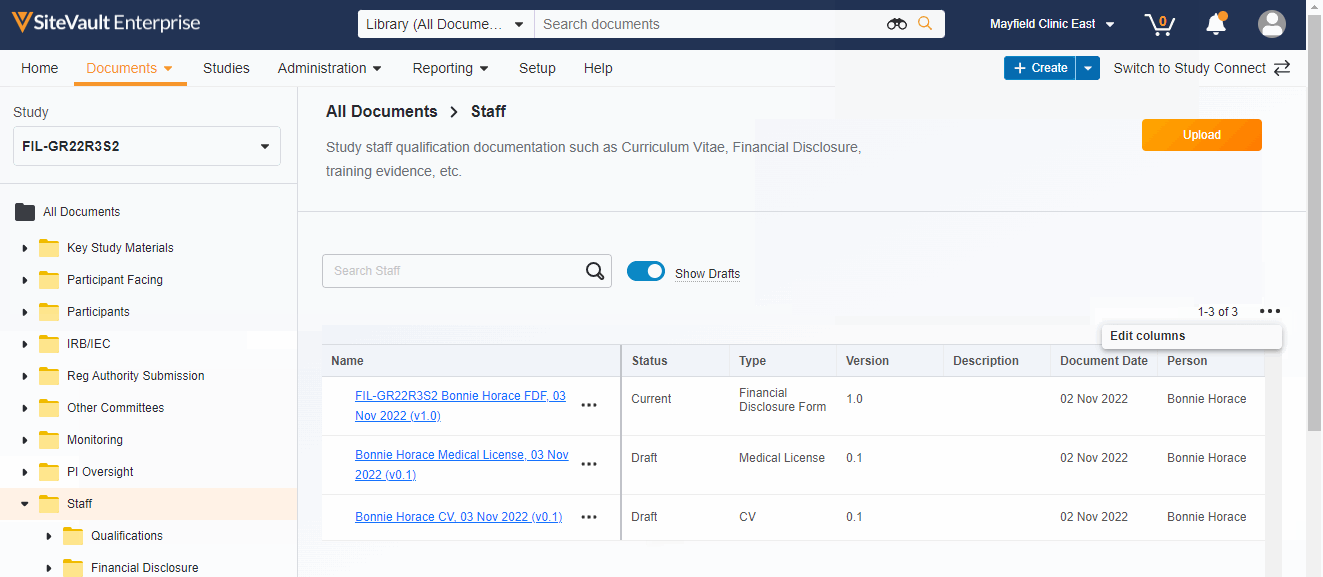
Study eBinder Redesign
This feature enhances the Study eBinder with a more modern look and feel. It also introduces the following usability enhancements:
- Document type-level folders
- Descriptions of document folders and document types
- The system displays help information when you position your pointer over certain elements in the Study eBinder
- Ability to display or hide draft documents
- Documents can be uploaded directly to the Study eBinder using a new Upload button or by dragging a document into a folder
Monitor & External User Access
With this feature, adding a monitor or external user to a specific site or study has been streamlined into a single process. You can now create accounts for your monitors and give them access to your studies all in the new Monitors & External Users tab.
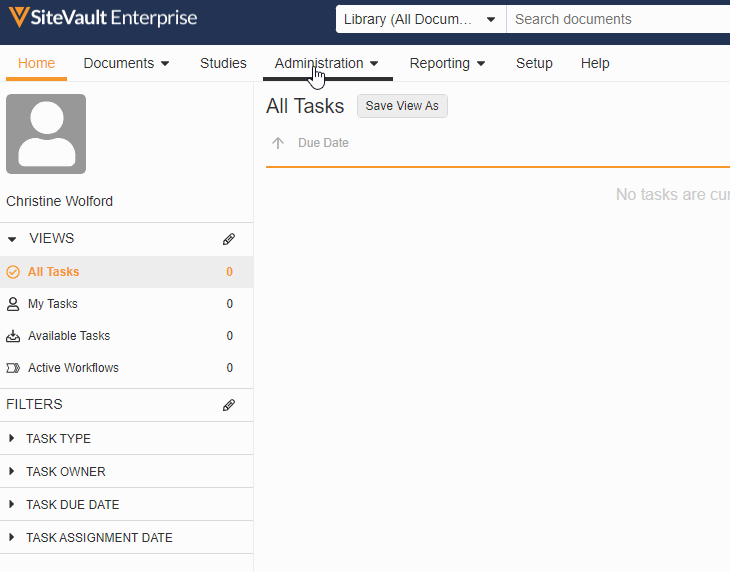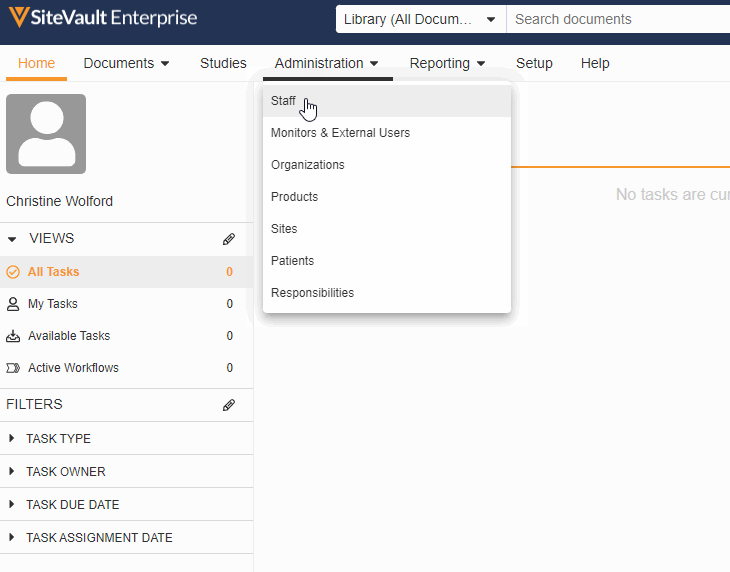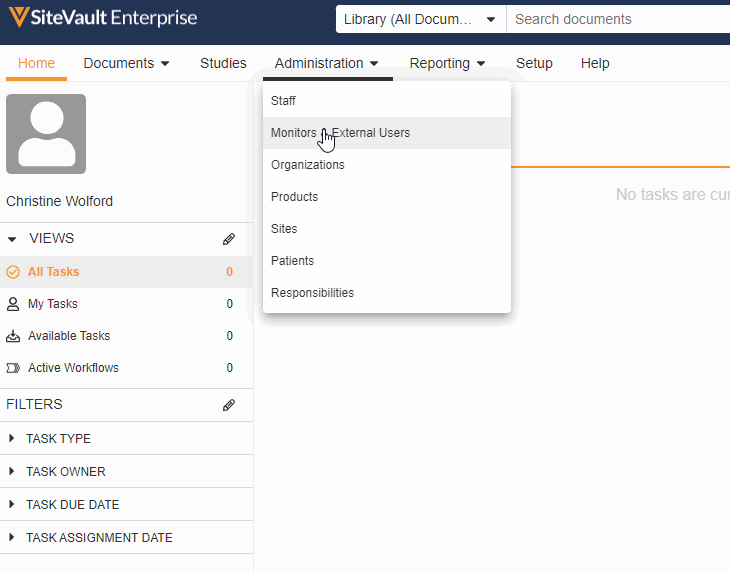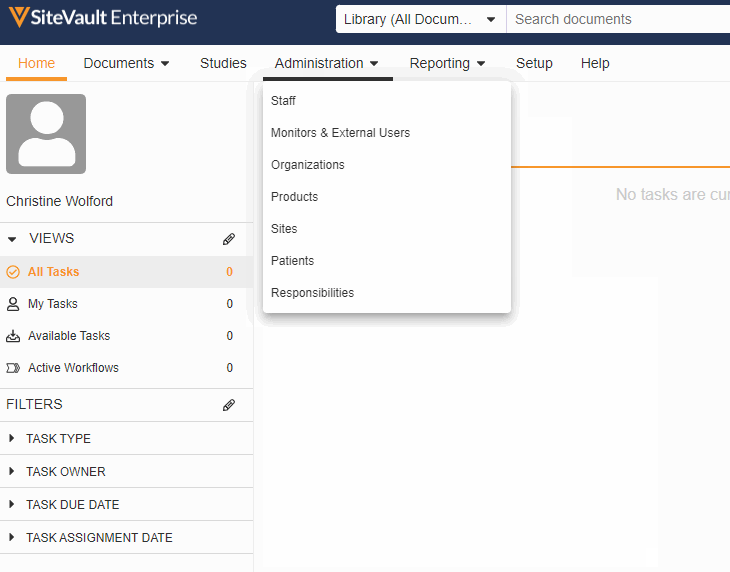
As part of this change, we’ve updated the name of the Profiles tab to Administration and the name of the Users tab to Staff and removed references to external users from the Staff and Setup Assistant pages. We’ve also made the following miscellaneous updates:
- Study Assignment Fields Renamed
- Access Start Date is updated to Scheduled Access
- Start Access End Date is updated to Scheduled Access End
- Lifecycle State is updated to Assignment Status
- Status is updated to Record Status
- All active user profile records are automatically updated to reflect the following:
- External User Profiles
- User Profiles is updated to Monitor/External User
- Updated page layouts and default list columns
- The Organization field is displayed on monitor or external user profiles
- Internal User Profiles
- User Profiles is updated to Staff
- Updated page layouts and default list columns
- External User Profiles
Digital Delegation: Support for Site Staff without User Accounts
This feature relaxes data validation behavior on Digital Delegation studies by granting more flexibility in managing users without SiteVault accounts, and by allowing users to initiate PI Approval when one or more staff member acceptance is outstanding.
For more information, see Digital Delegation.
Update eConsent Workflows to Support Paper-Signed Forms and Additional Review
Note This feature is available in both SiteVault eRegulatory and SiteVault Study Connect for Veeva eConsent or Veeva ePRO customers.
This feature makes the following enhancements to the eConsent countersignature workflow:
- For paper-signed eConsent forms uploaded to SiteVault, you can now certify as copy and countersign the form in SiteVault.
- When countersigning an eConsent form, you can now assign an additional reviewer to review the form.
Reason for Change Required for Specific Participant-Related Updates
Note This feature is available in both SiteVault eRegulatory and SiteVault Study Connect for Veeva eConsent or Veeva ePRO customers.
With this feature, the system requires you to enter a reason for change when you update a participant ID or the actual date and time on participant events. This information is documented in the system’s audit trail.
For additional information on this exciting release, please visit the SiteVault 22R3 Release Hub.
Veeva eConsent
eConsent - Study Connect Available Dec 3, 2022
This feature allows site users to use Veeva eConsent in Study Connect. Study Connect streamlines the eConsent workflow for connected studies by allowing site users to manage sponsor-created template eConsent forms, create and manage profiles for study participants and their signatories, send and countersign eConsent forms, and view eConsent response data in one centralized app. Users can access the Veeva eConsent editor from blank eConsent templates in Study Connect, reducing the amount of time and navigation required to prepare eConsent forms for signature.
eConsent in Study Connect can be used by sites that are invited to an eConsent-enabled connected study by a sponsor. Site users that are on an eConsent-enabled connected study prior to 22R3 can continue using eConsent in SiteVault, but we recommend using Study Connect for a streamlined connected study experience. Notification links for connected studies will also be redirected to Study Connect.
For more information, see the following help topics:
- Configuring Veeva eConsent Authoring (Clinical Operations) in Clinical Operations Help
- Overview of Veeva eConsent in Study Connect Help
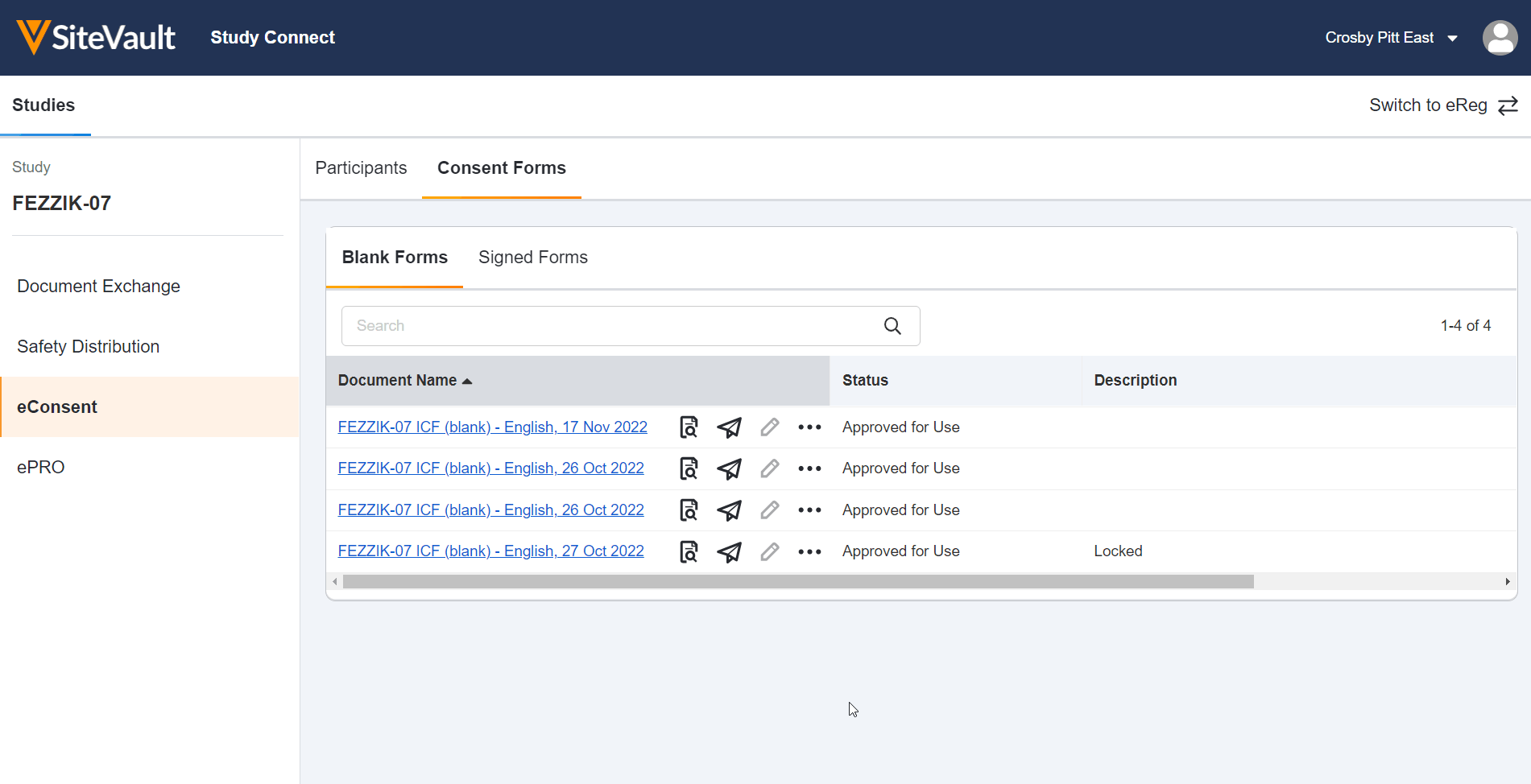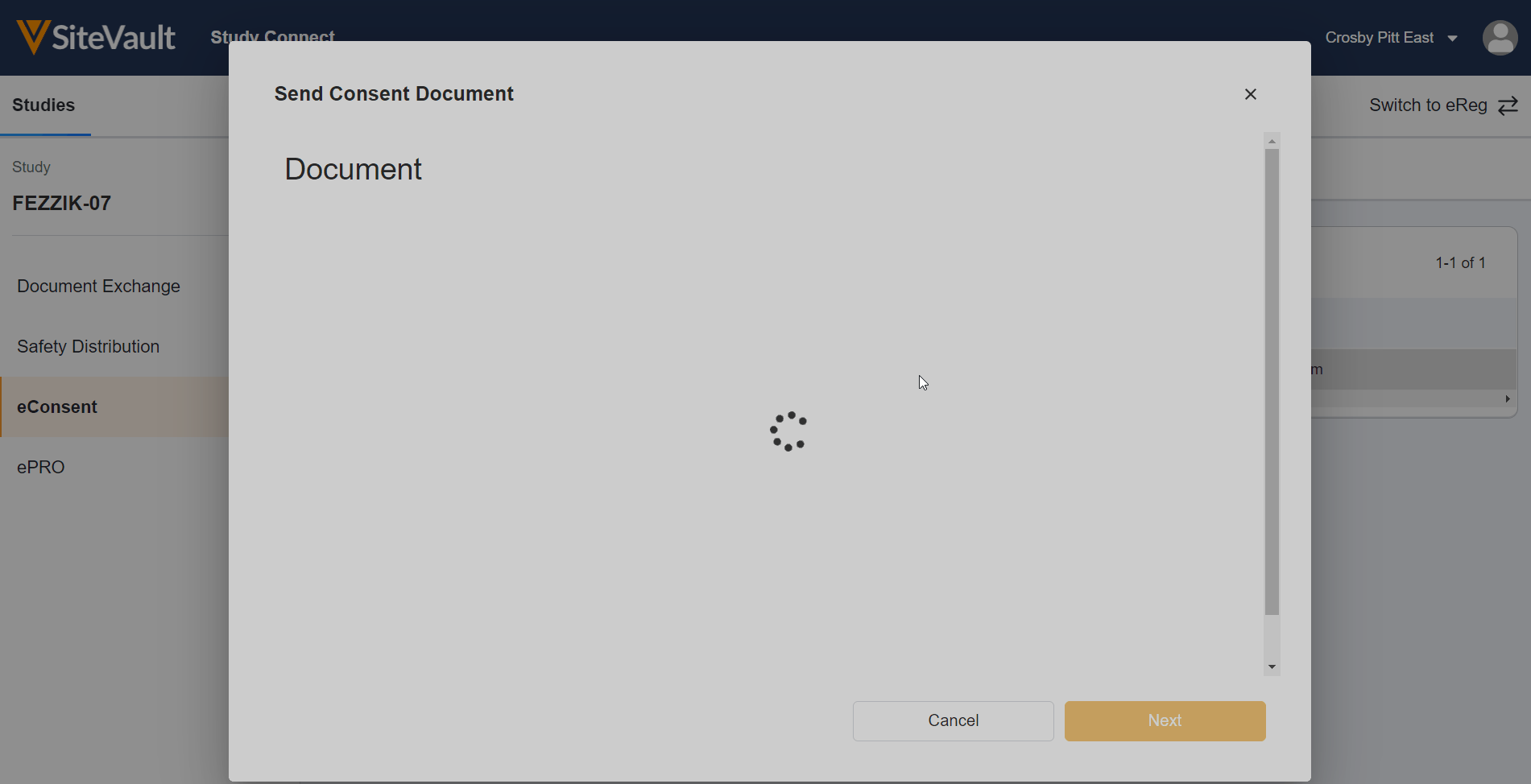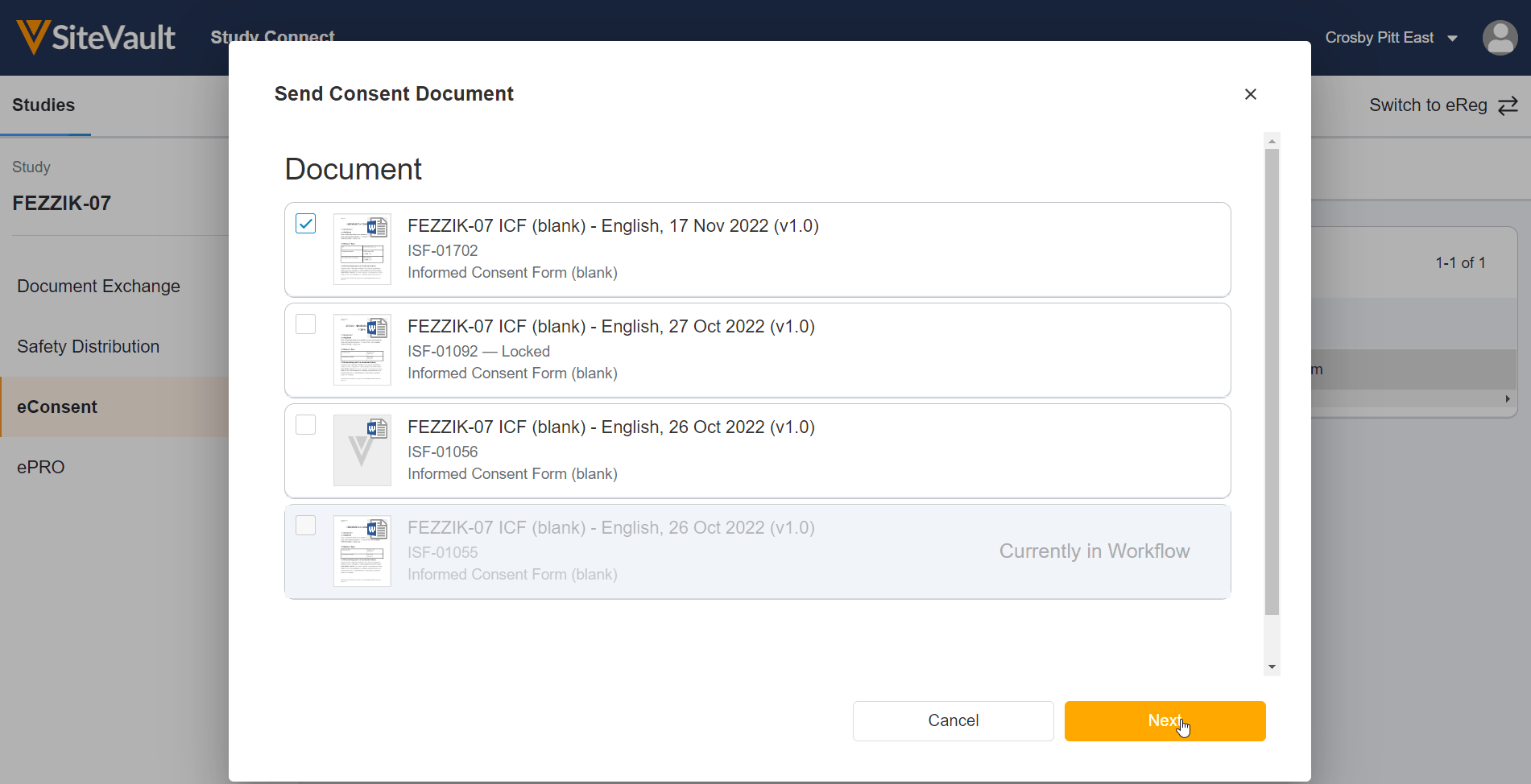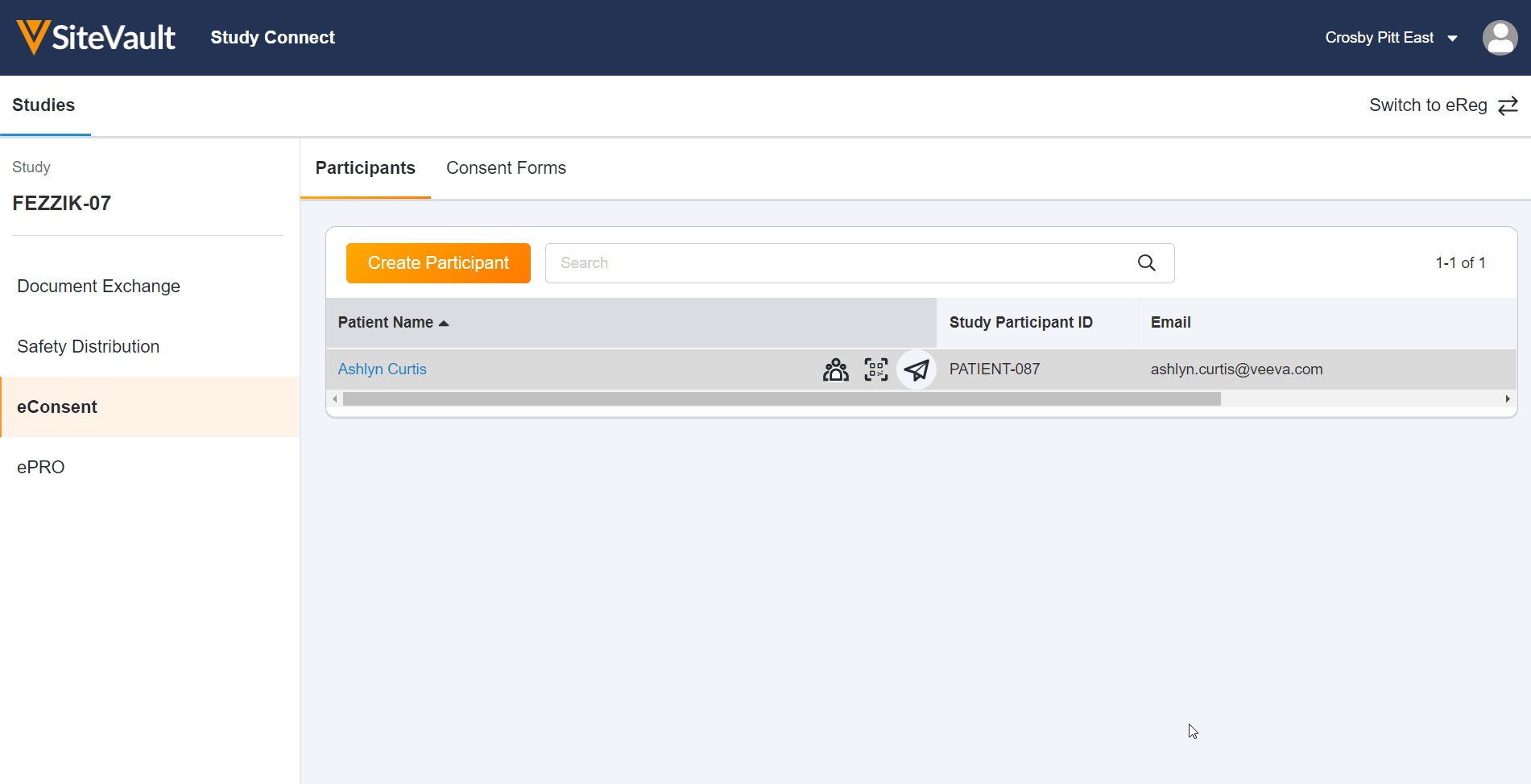
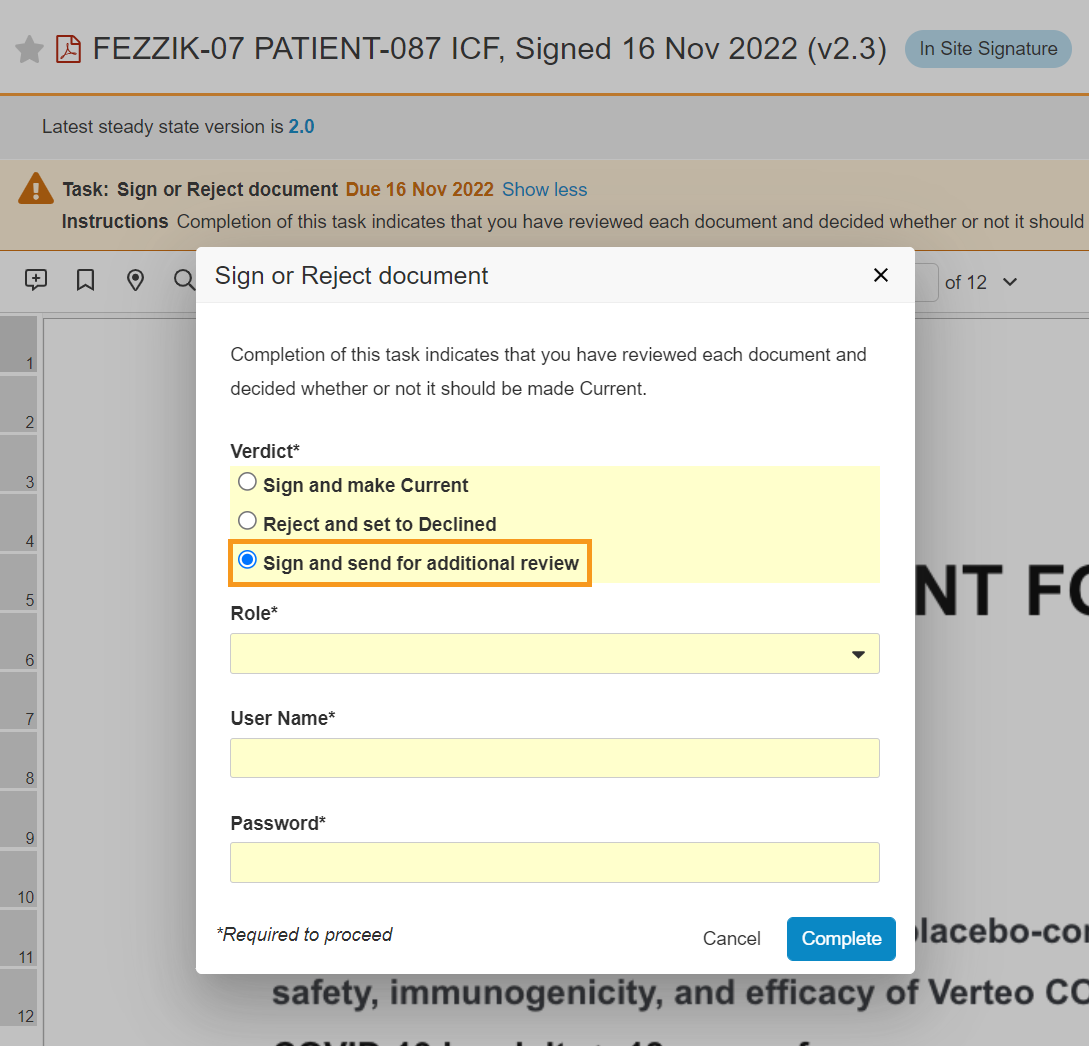
Update eConsent Workflows to Support Paper Signed Forms and Additional Review
This feature makes the following enhancements to the eConsent countersignature workflow:
- SiteVault users can print eConsent forms for participant wet-ink signature on paper to upload, certify, and countersign in SiteVault.
- When countersigning an ICF, SiteVault users can assign an additional reviewer to review the form.
Connected eConsent Forms Only Via Clinical Distribution
Sponsor users must now distribute eConsent forms to sites from Vault Clinical in order to receive response data. Site users can no longer create sponsor-connected documents via a share code or Microsoft Word import.
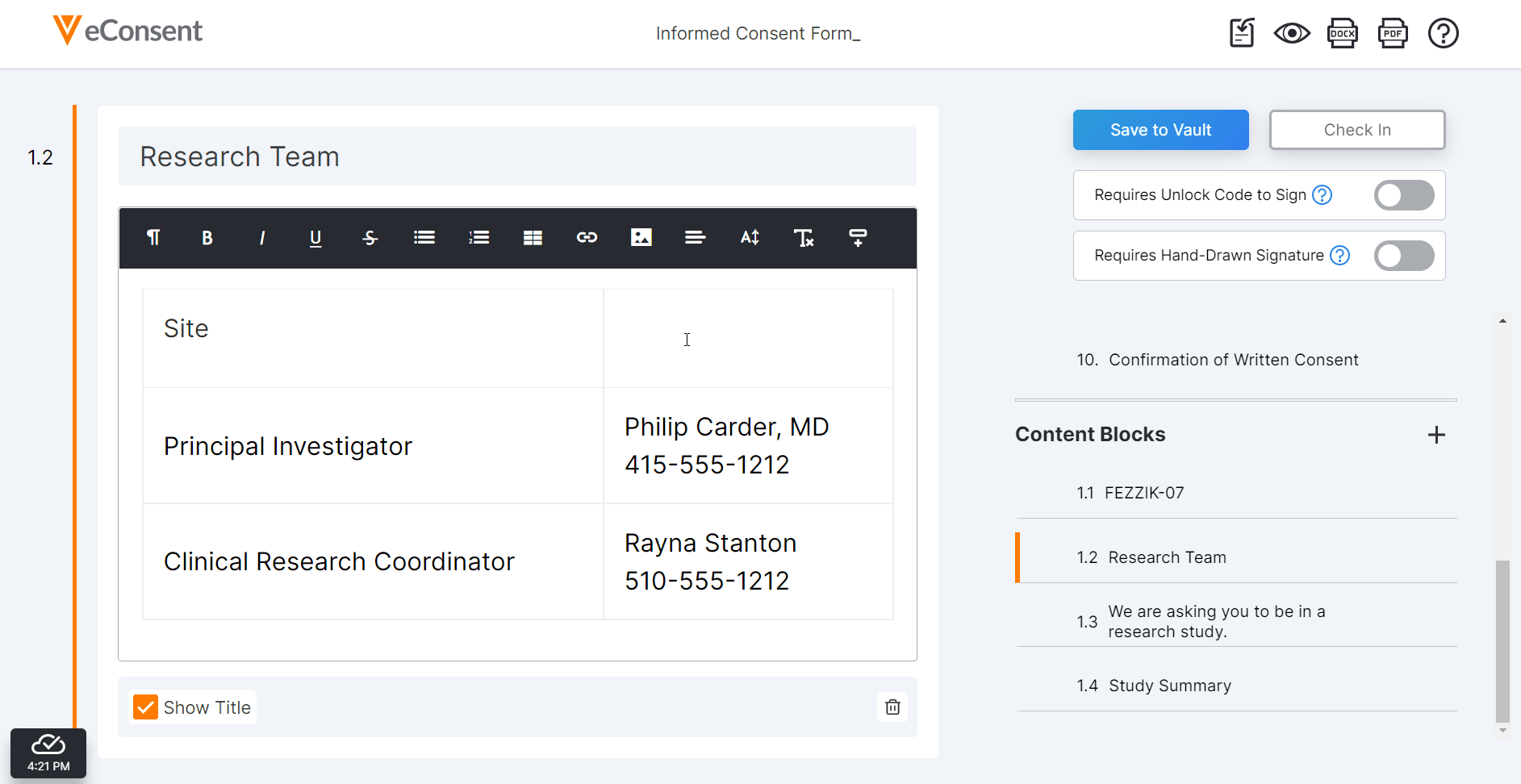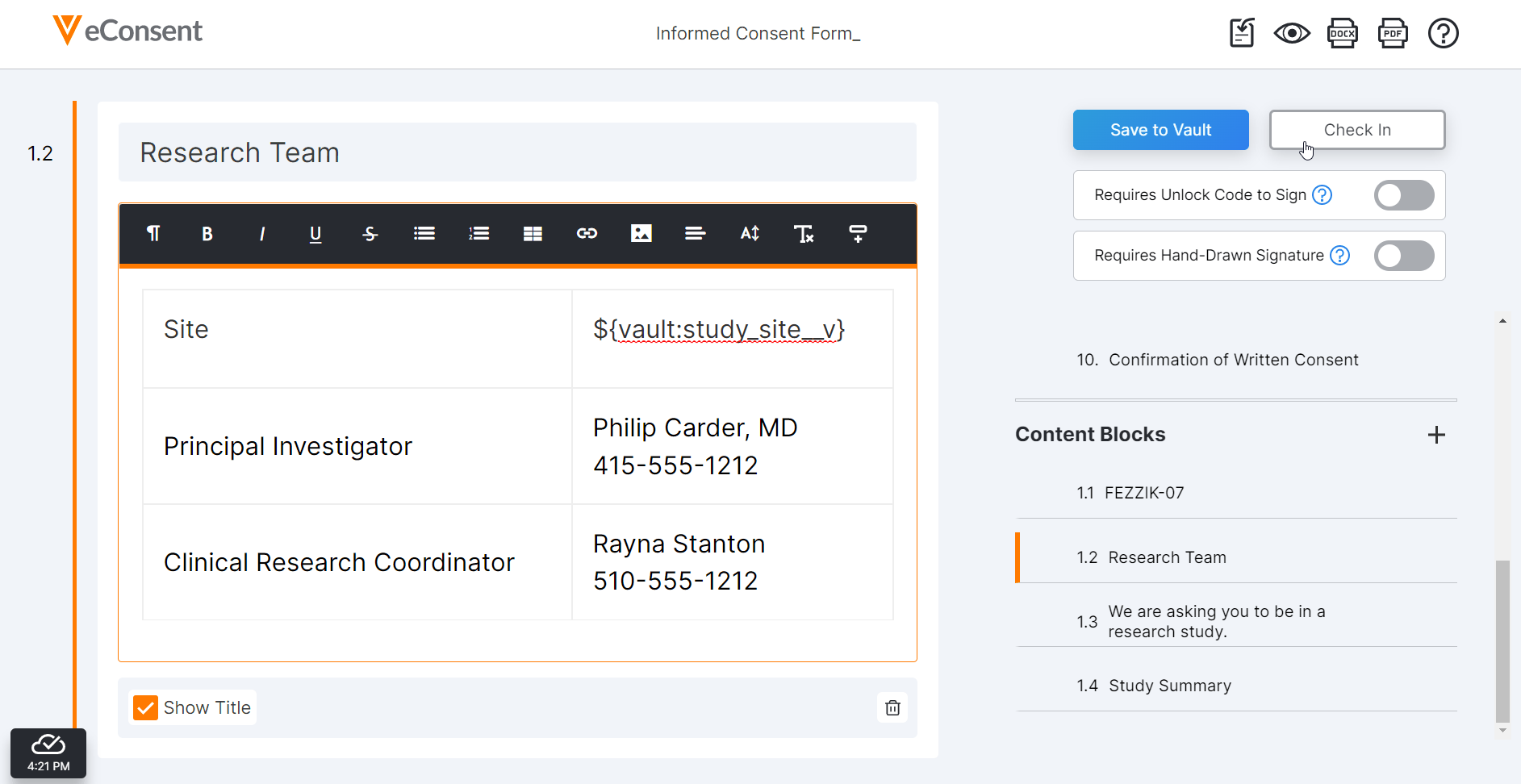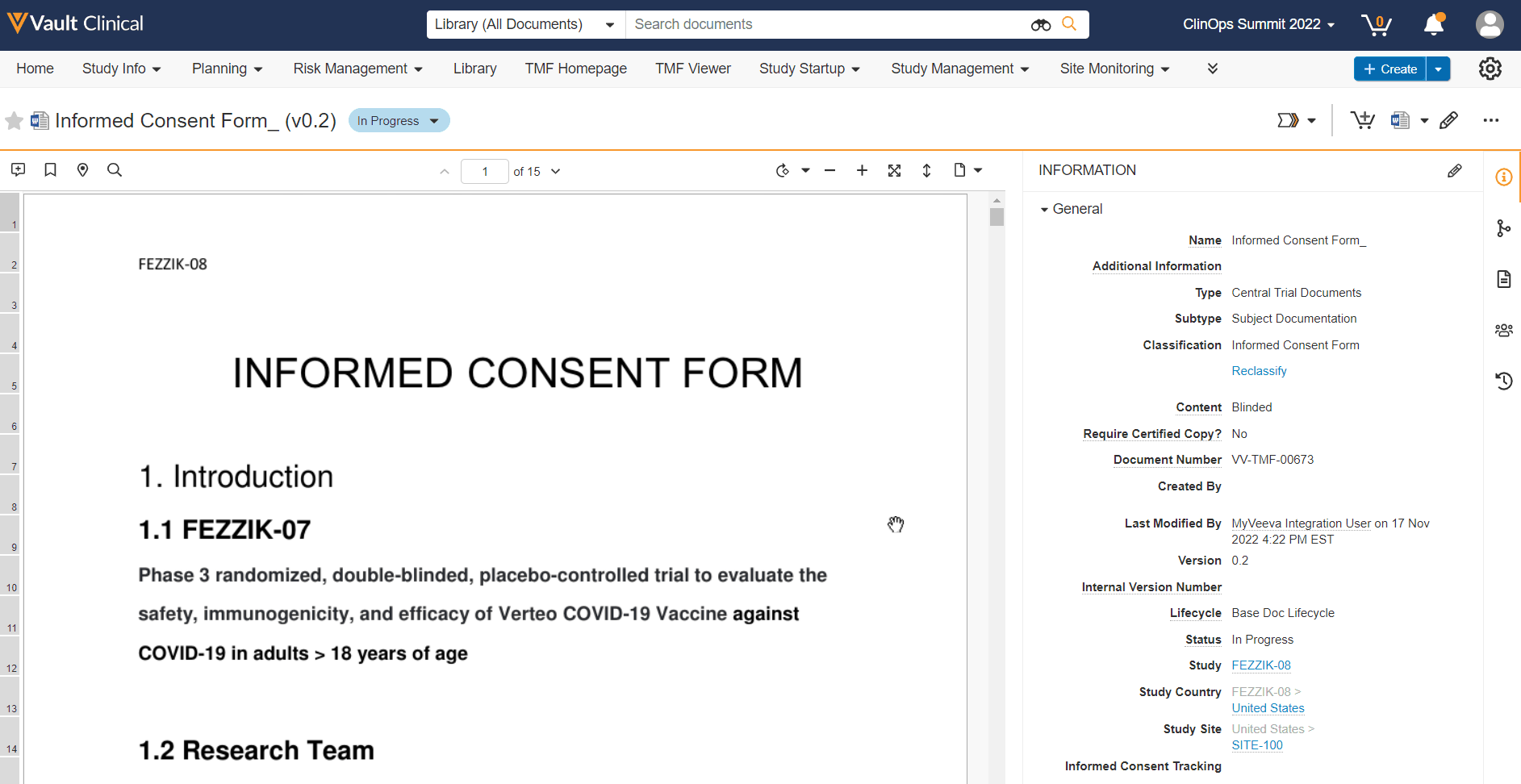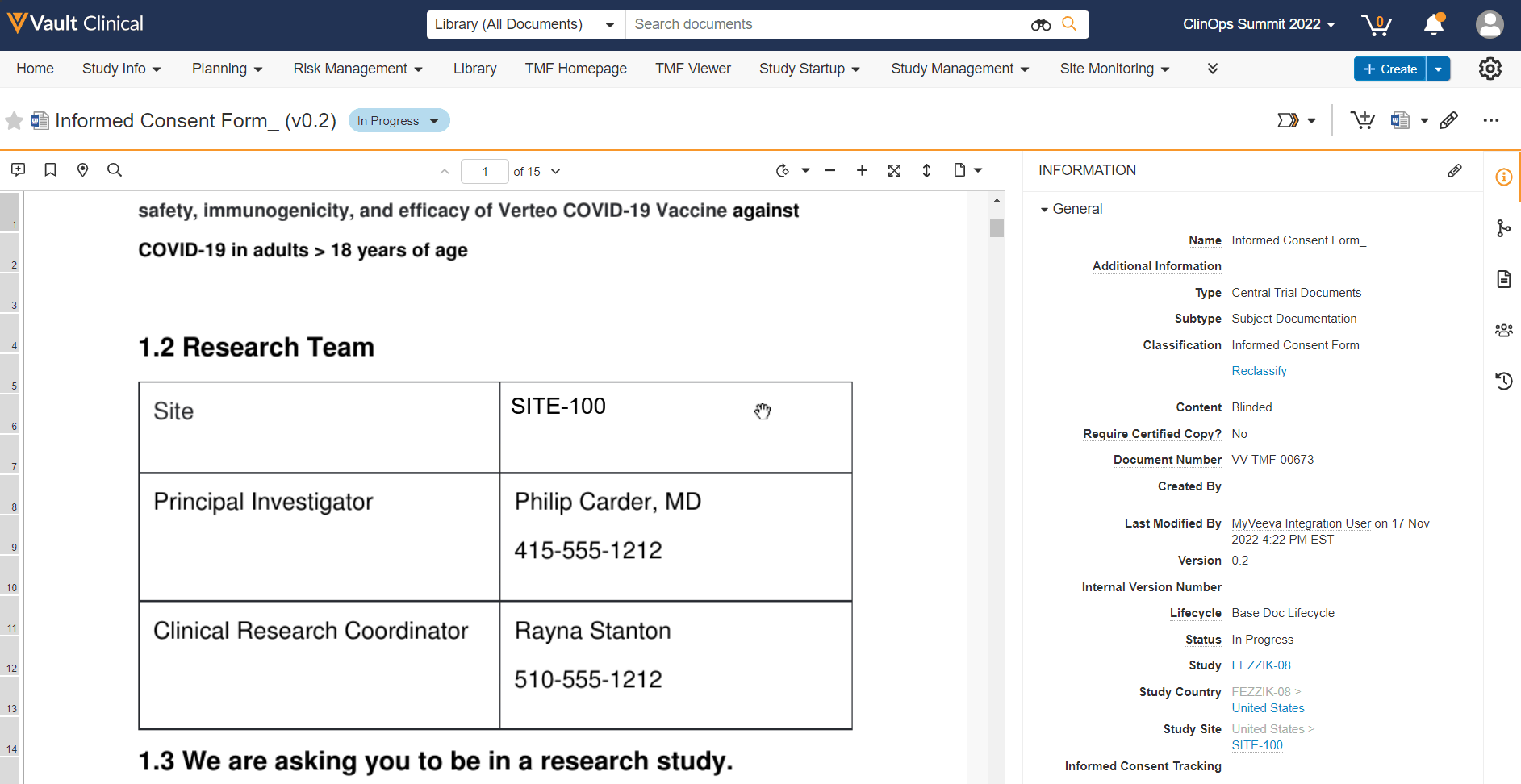
eConsent Merge Fields
This feature allows sponsor users to use Vault merge fields in eConsent forms and automatically import variable text values from Clinical Operations to a template eConsent document, reducing the need to manually edit individual copies of the template for specific studies, sites, and countries. Sponsors using a Microsoft Word-based eConsent template can add merge fields to the document in Microsoft Word and import it to the eConsent editor. Additionally, users can add merge fields to the document in the eConsent editor.
For more information, see Using the Veeva eConsent Editor in Clinical Operations Help.
Header and Footer in Blank Word-Based ICFs
When site staff check in a Microsoft Word-based eConsent form to Vault from the eConsent editor, the system now applies the same header and footer to the .DOCX file that’s applied to .PDF files. Previously, the system didn’t apply this header or footer to .DOCX files.
This feature was released in the 22R2.0.3 maintenance release on September 16, 2022.
For more information, see Using the Veeva eConsent Editor in Clinical Operations Help.


eConsent Sponsor Templates
With this update, Study- and Country-level eConsent documents must be designated as a Sponsor Template before sending to Sites. This designation is made using the new Sponsor Template for Site field located in the Site Information section of the document.
For more information, see the Exchanging eConsent Forms (Veeva Site Connect) section in Clinical Operations Help.
Veeva ePRO
ePRO in Study Connect Available Dec 3, 2022
This feature allows site users to use Veeva ePRO in Study Connect. Study Connect streamlines the ePRO workflow for sites and allows users to approve a study’s ePRO collection document, manage a study’s ePRO languages, create and manage study participants, enable and disable ePRO for study participants, and manage participant events in one centralized app. Users can also view ePRO-related notifications in their notification bell on the ePRO tab. In Study Connect, users can export a study’s ePRO data and automatically receive new ePRO collection documents from a sponsor in the event of an upversioned ePRO collection.
ePRO in Study Connect can be used by sites that are invited to an ePRO-enabled connected study by a sponsor. Site users that are on an ePRO-enabled connected study prior to 22R3 can continue using ePRO in SiteVault, but we recommend using Study Connect for a streamlined connected study experience. Notification links for connected studies will also be redirected to Study Connect.
See the following help topics for more information:
- Using Veeva ePRO in Clinical Vault in Clinical Operations Help
- Overview of Veeva ePRO in Study Connect Help
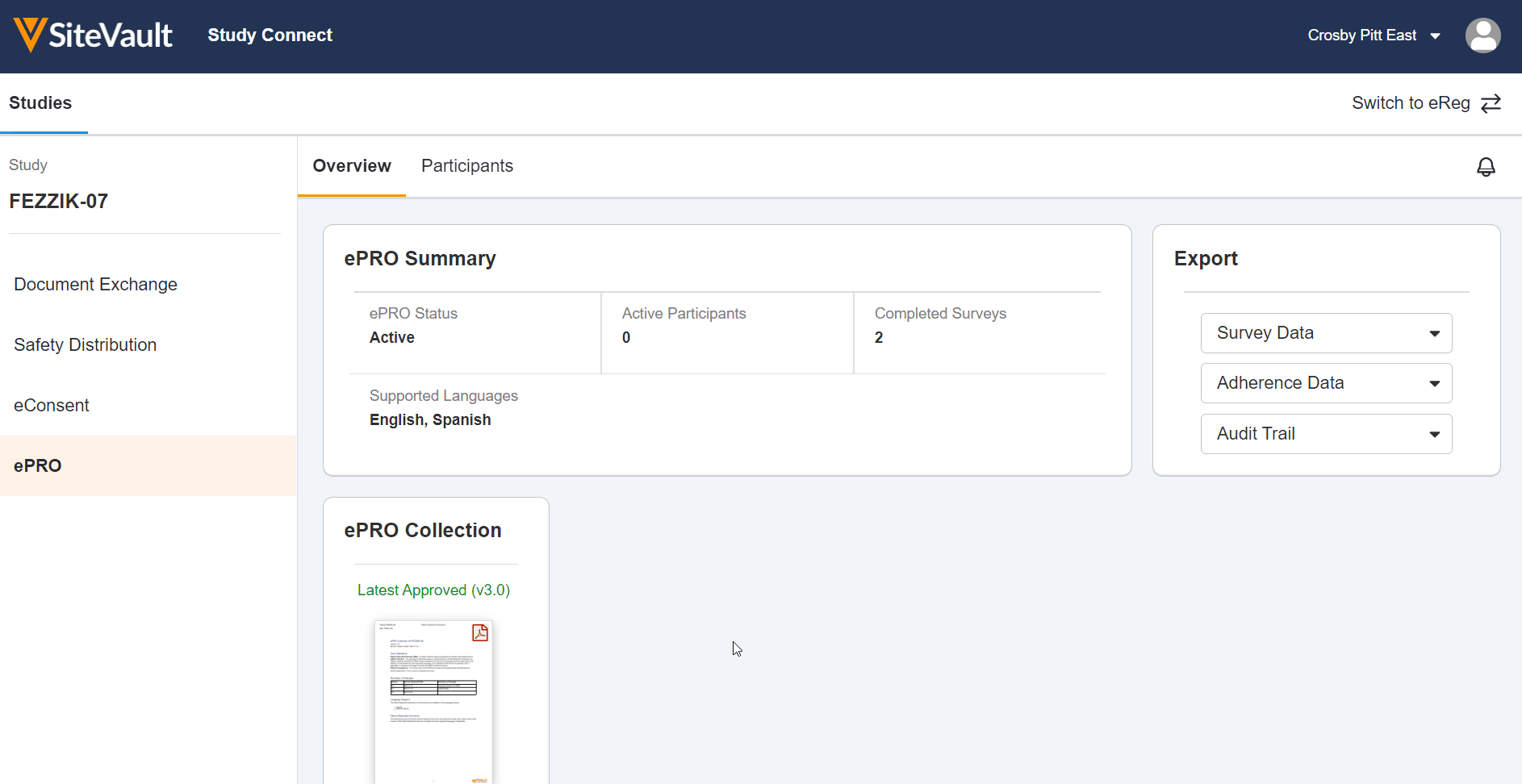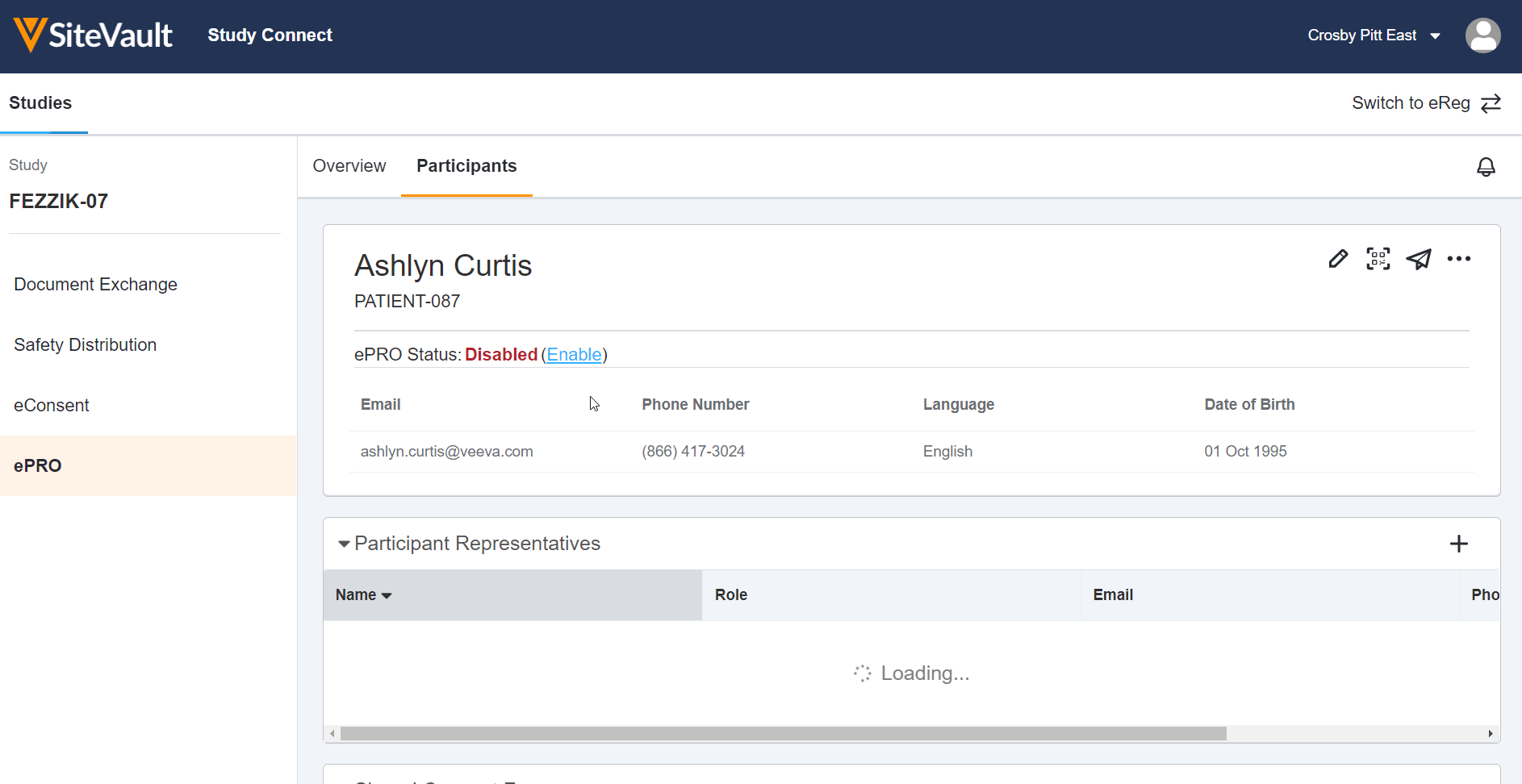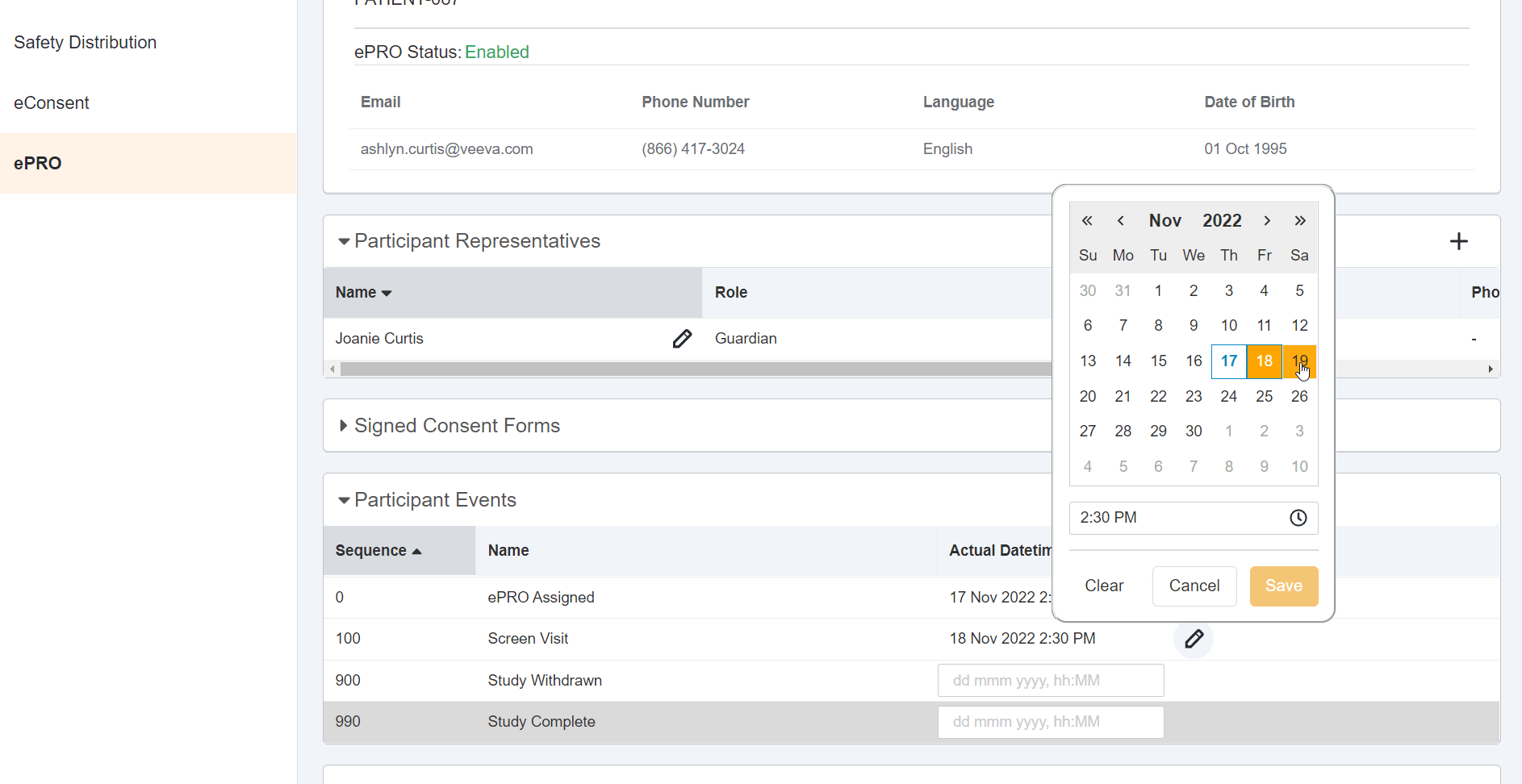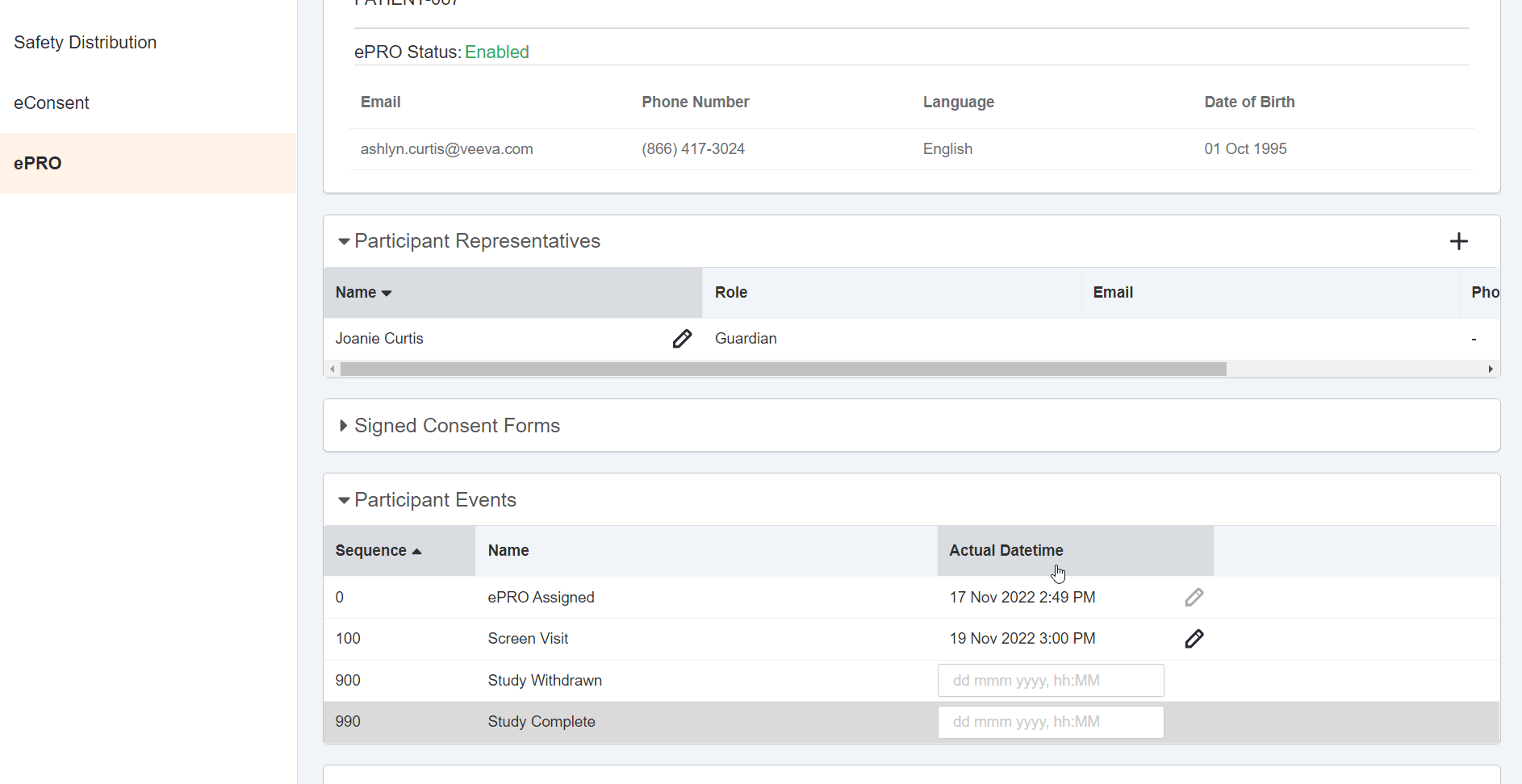
Date, Time, or Datetime Survey Component
This feature allows sponsor users to configure a survey that contains a date, time, or datetime entry question for MyVeeva users to complete. Sponsor users may configure minimum, maximum, and default values for responses to date, time, and datetime entry questions. These values may be static (such as December 02, 2022 at 23:59) or dynamic (such as -7 days from the current date when the MyVeeva user is responding). Sponsor users can also configure new survey condition capabilities that support branching a survey based upon the values that participants enter when responding to date, time, or datetime entry questions.
See the following help topics for more information:
- Configuring a Date Entry Question Type in Clinical Operations Help
- Configuring a Time Entry Question Type in Clinical Operations Help Help
- Configuring a Datetime Entry Question Type in Clinical Operations Help
- Surveys in MyVeeva for Patients Help
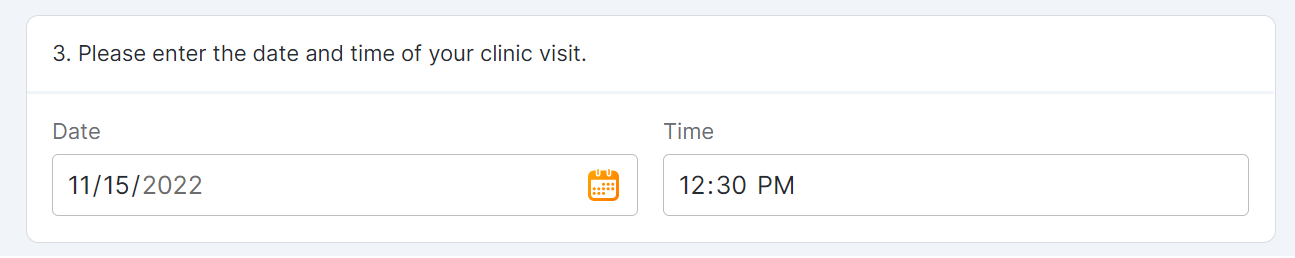
Number Entry Survey Component
This feature allows sponsor users to configure a survey that contains a number entry question for MyVeeva users to complete. Sponsors must configure a minimum value, maximum value, and increment value for responses. Sponsor users can also configure new survey condition capabilities that support branching a survey based upon the values that participants enter when responding to number entry questions.
See the following help topics for more information:
- Configuring a Number Entry Question Type in Clinical Operations Help
- Surveys in MyVeeva for Patients Help
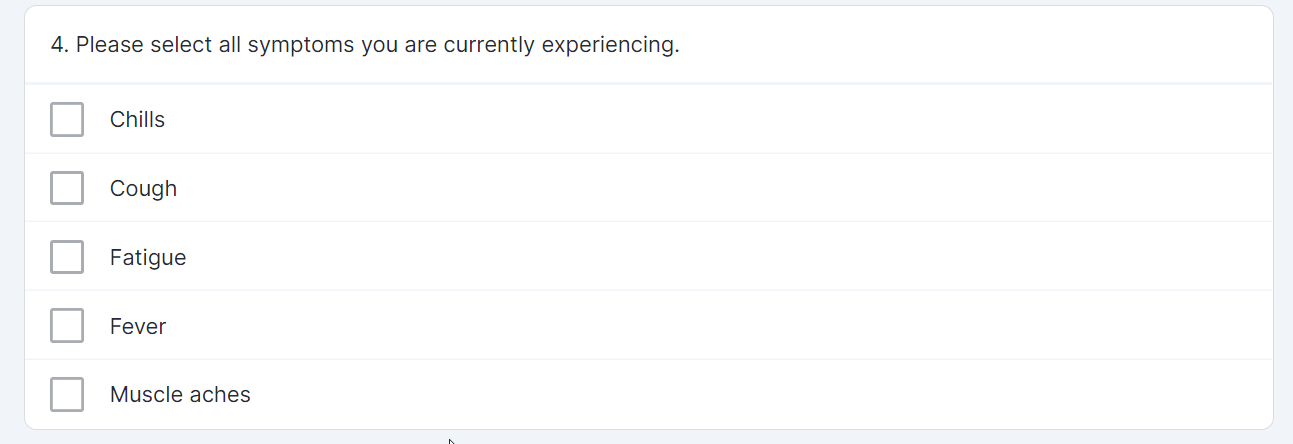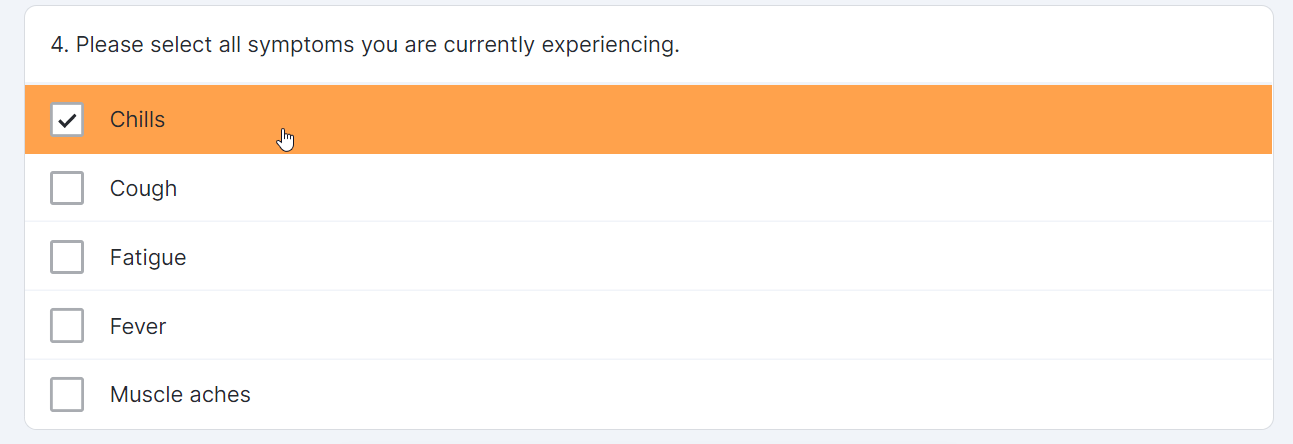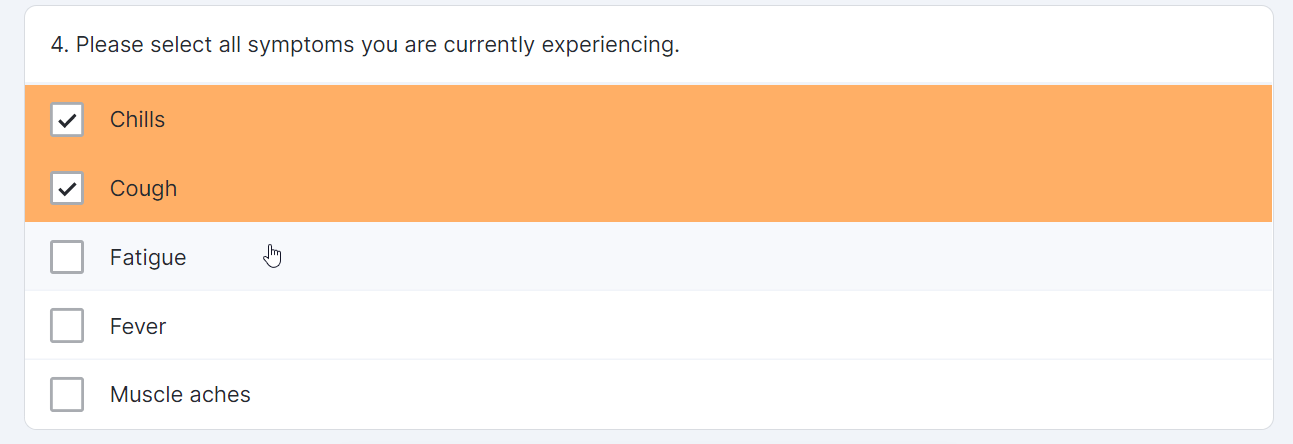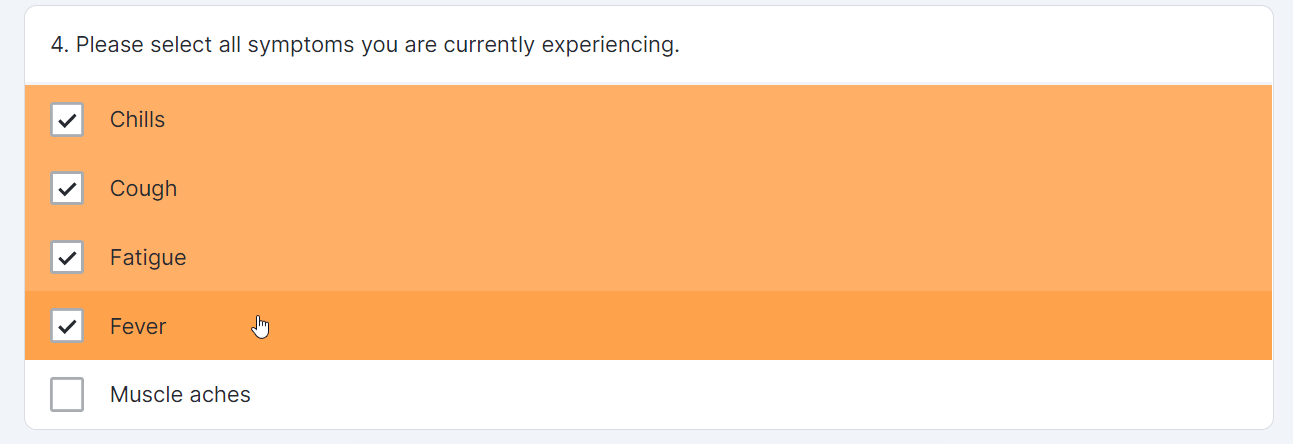
Multiple-Choice Survey Component
This feature allows sponsor users to configure a survey that contains a multiple-choice question for MyVeeva users to complete. Sponsor users can also configure new survey condition capabilities that support branching a survey based upon the values that participants select when responding to multiple-choice questions.
See the following help topics for more information:
- Configuring a Multiple-Choice Question Type in Clinical Operations Help
- Surveys in MyVeeva for Patients Help
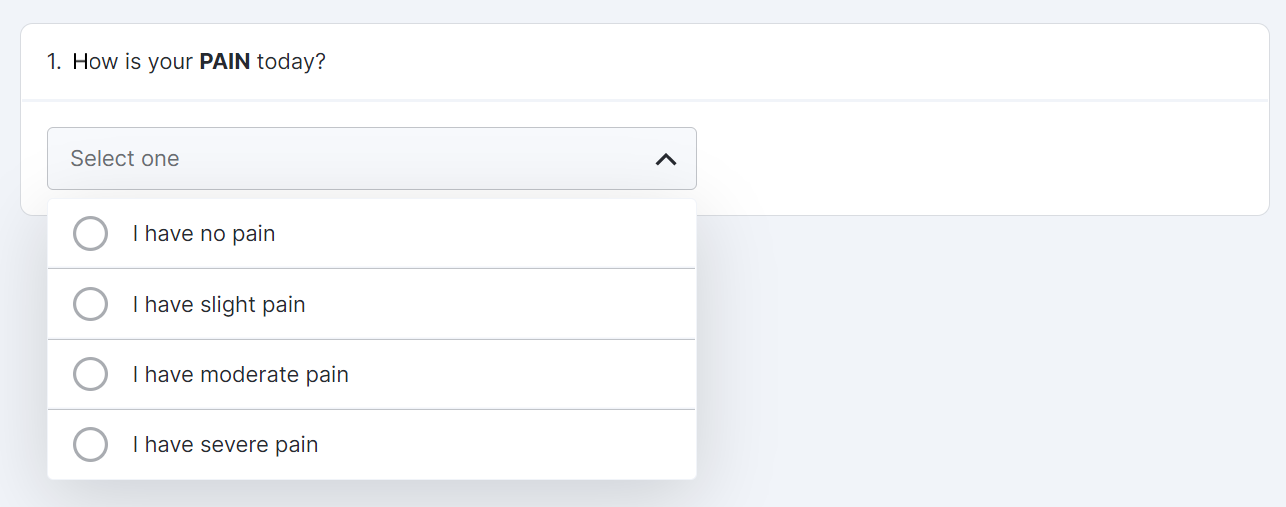
Single-Choice Drop Down Survey Component
This feature allows sponsor users to configure a survey that contains a single-choice question with responses displayed in a drop-down menu for MyVeeva users to complete.
See the following help topics for more information:
- Configuring a Single-Choice/Verbal Rating Scale (VRS) Question Type in Clinical Operations Help
- Surveys in MyVeeva for Patients Help
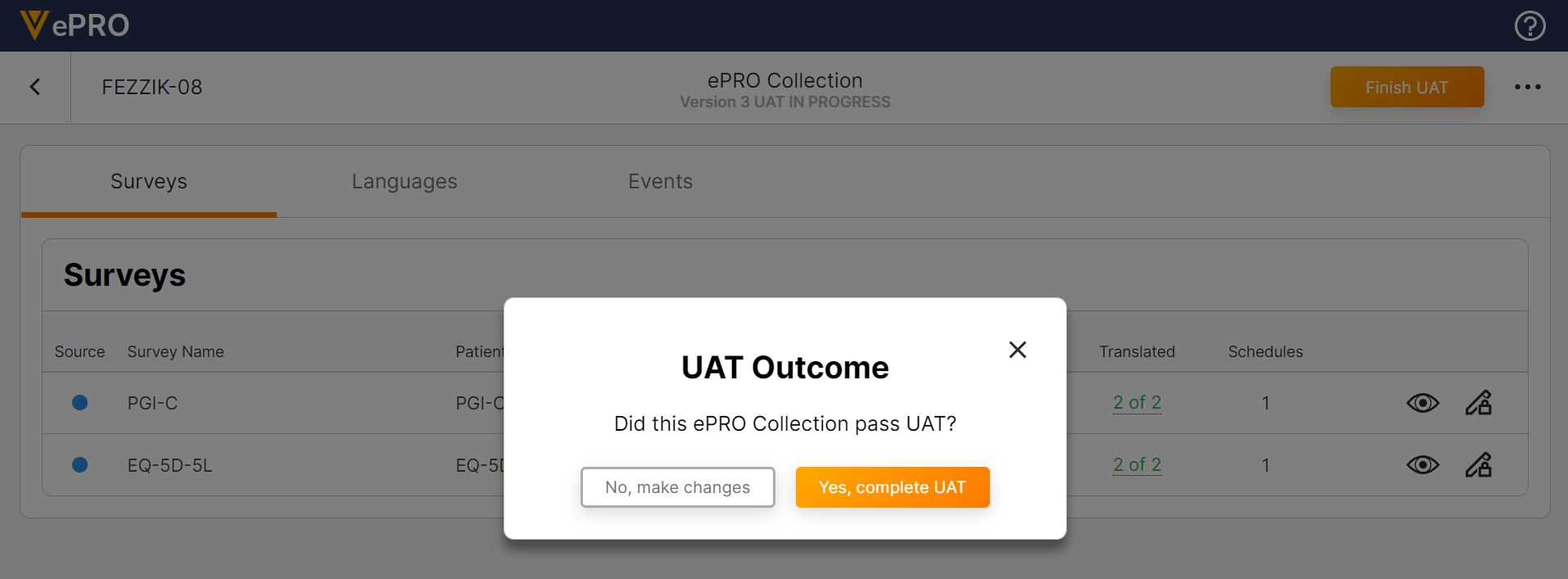
ePRO UAT Process
This feature allows sponsor users to easily set up a testing environment to complete user acceptance testing (UAT) on ePRO collections. Once a user starts UAT for their ePRO collection, they can connect their study build to a study in a sandbox SiteVault environment by entering the UAT ePRO code in SiteVault. Once connected, sponsor users can perform all site and participant user actions in an environment similar to a production environment.
Sponsor users can export all survey and adherence ePRO data captured during UAT. Users can choose to make changes after UAT and resume editing the ePRO collection, or they can choose to complete UAT and approve the ePRO collection. Users can complete UAT on new and upversioned ePRO collections.
For more information, see Performing User Acceptance Testing (UAT) on an ePRO Collection in Clinical Operations Help.
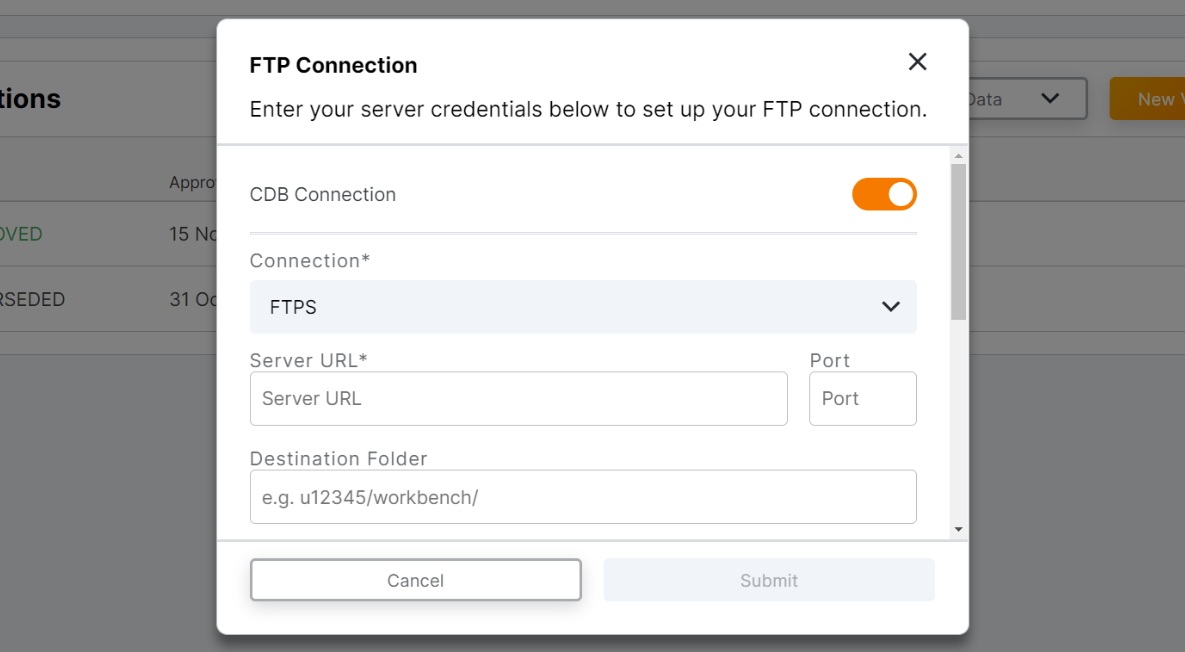
ePRO FTP Connection
This feature allows sponsor users to create an FTP connection via FTPS in the Veeva ePRO module to automatically send ePRO data to Veeva CDB or aun external FTP supporting FTPS. Users can create an FTP connection in the ePRO module by entering their Veeva CDB or external server credentials, selecting which ePRO data sets are sent, and testing the connection. Once an FTP connection is established, the entire ePRO data set is sent once per day.
for more information, see the Creating an FTP Connection to Automatically Transfer ePRO Data section in Clinical Operations Help.
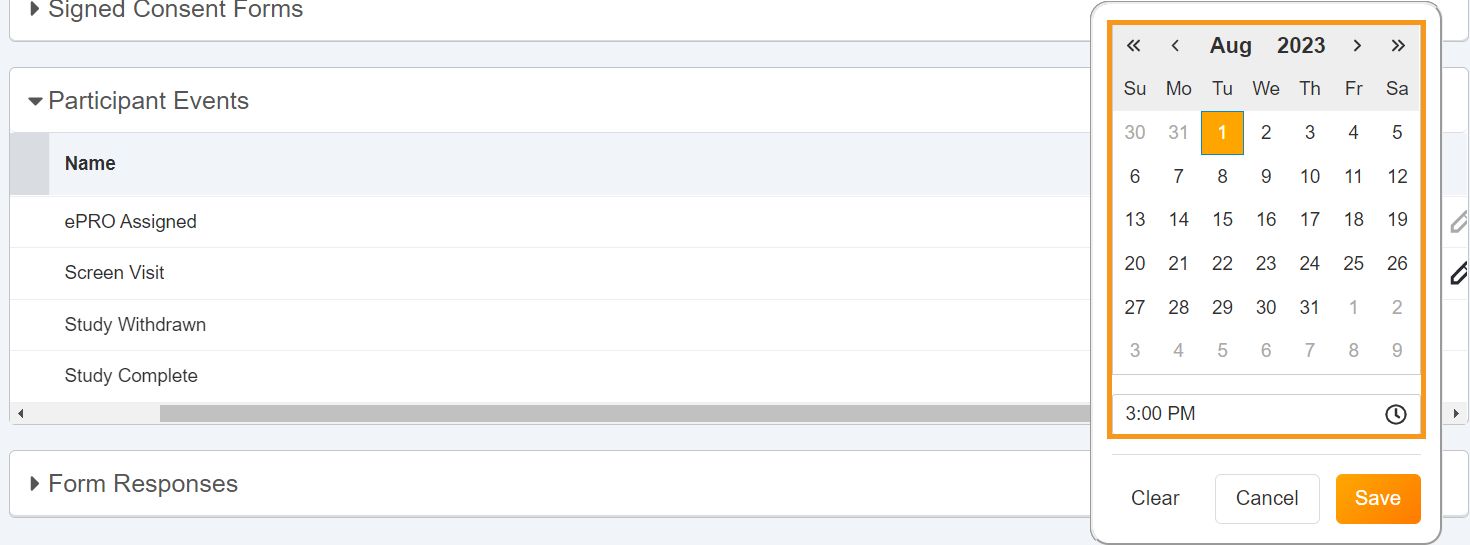
Support Future Event Datetimes & Negative Delays
This feature allows sponsor users to use a negative delay in ePRO schedules to send surveys to participants prior to future events. As site users add or update participant event datetimes to be in the future, ePRO schedules are rescheduled as applicable.
See the following help topics for more information:
- Managing Events in Clinical Operations Help
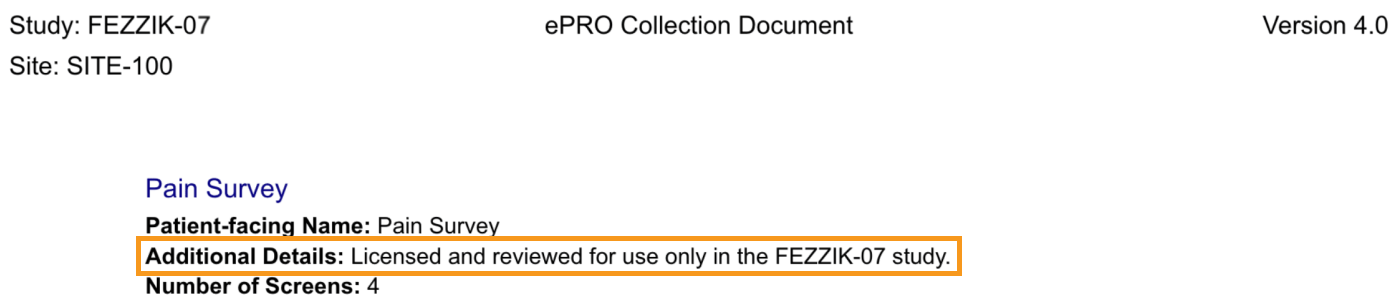
Add Additional Details to ePRO Collection Document
This feature allows sponsor users to add additional details to a survey. Additional details appear on the ePRO collection document and are only visible to sponsor and site users. Additional details don’t appear to participant users.
See the following help topics for more information:
- Universal Survey Parameters section in Clinical Operations Help
- Exporting a PDF Overview of an ePRO Collection section in Clinical Operations Help
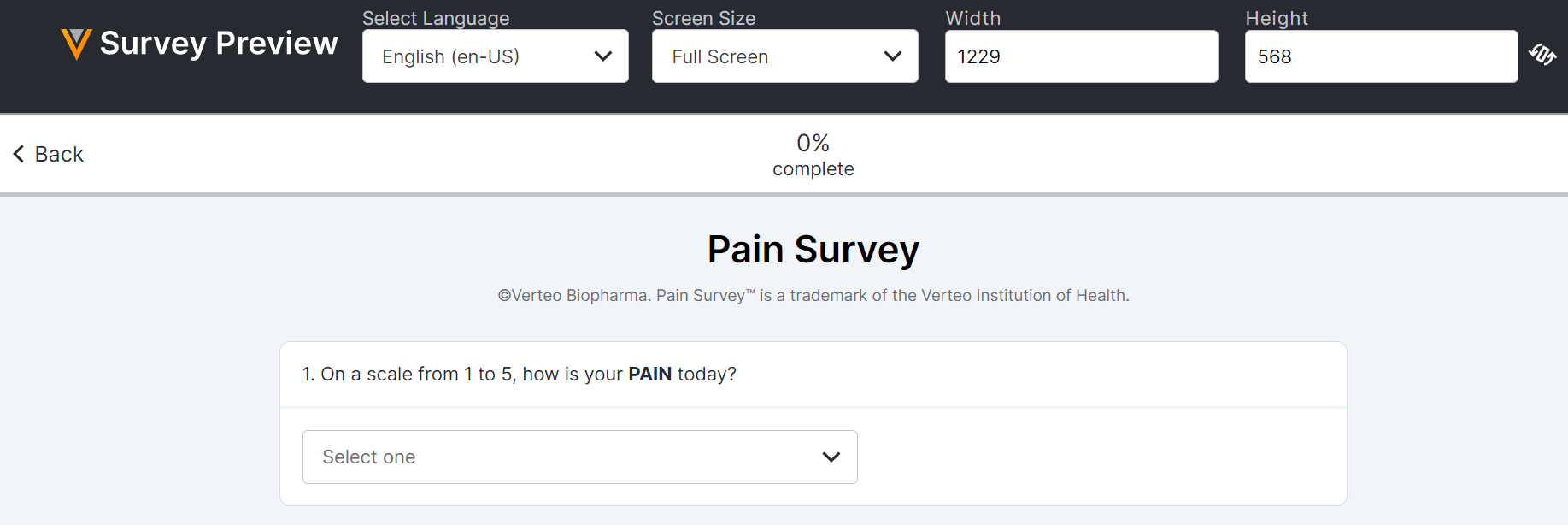
Survey Preview Enhancements
This feature improves the user experience when previewing surveys in the ePRO module. Users can preview a survey in a variety of phone, tablet, and laptop screen resolutions and in custom screen resolutions, as well as rotate the screen orientation.
For more information, see the Previewing a Survey section in Clinical Operations Help.
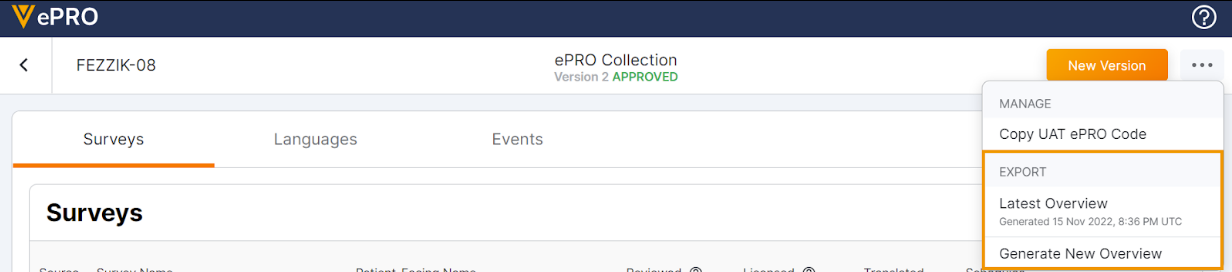
Generate ePRO Overview Asynchronously in ePRO Module
This feature allows sponsor users to create a new .PDF overview of an ePRO collection or to download a previous version in the ePRO module.
For more information, see the Exporting a PDF Overview of an ePRO Collection section in Clinical Operations Help.
Add Reason for Change to Audit Trail Data
This feature enhances the ePRO audit trail data exports for sponsor and site users by adding a Reason for Change column containing the reason an ePRO data change occurred. This column only populates if a site requests an ePRO data change and it’s processed by Veeva.
See the following help topics for more information:
- Exporting Survey, Adherence, and Audit Trail Data in Clinical Operations Help
Adherence Report Includes Available Surveys
This feature improves the site and sponsor ePRO adherence data exports to include a row of data for each survey that is currently available to MyVeeva users.
Adherence data exports now include an additional Available status in the Adherence column, which may impact any external post-export data loads that users have set up for data analysis.
See the following help topics for more information:
- Exporting Survey, Adherence, and Audit Trail Data in Clinical Operations Help
Survey Complete Site Notification
This feature allows sponsor users to configure a notification to be sent to site users in SiteVault and Study Connect when a participant completes a survey task.
See the following help topics for more information:
- Configuring Survey Complete Notifications in Clinical Operations Help
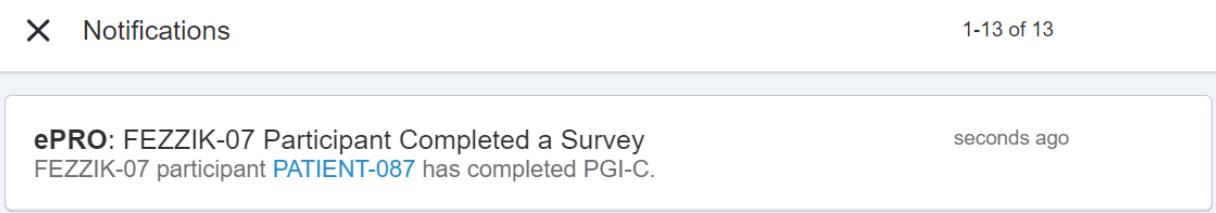
Additional ePRO Site Notifications
This feature allows site users to receive new ePRO-related notifications in SiteVault and Study Connect.
New notifications related to participants alert site users when:
- enabling a participant with ePRO is successful
- disabling ePRO from a participant is successful
Additional new error notifications alert users when:
- attempting to export survey, audit trail, or adherence data results in an error
- approving a new ePRO collection document is successful
- disconnecting ePRO from a study either is successful or results in an error
- updating the supported languages for a study’s ePRO either is successful or results in an error
See the following help topics for more information:
- Configuring Schedules and Notifications in Clinical Operations Help