Certified Copy of a Document Definition: “A copy (irrespective of the type of media used) of the original record that has been verified (i.e., by a dated signature or by generation through a validated process) to have the same information, including data that describe the context, content, and structure, as the original.” - ICH Guideline for GCP
SiteVault provides the option for documents to be classified as certified copies. It is up to the site/organization to determine which documents are required to be certified copies. A document is considered a copy of source if it has been downloaded from an electronic source or electronic health record (EHR) system or if it has been scanned before being uploaded to SiteVault. A document is considered an original source document if this is the file where the data was originally captured (for example, if a .DOCX file was completed during the visit).
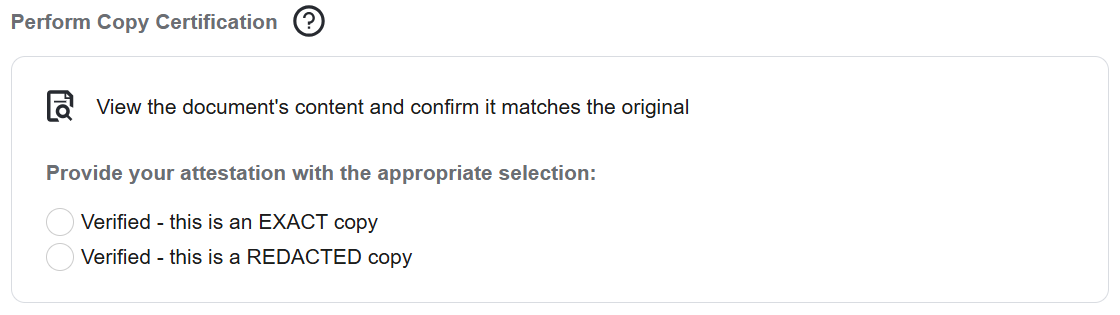
Certify as a Copy
A document can be certified as a copy during upload, while approving (finalizing), or after approval.
- Certify During Upload
- Certify During Finalization
- Certify After Finalization (approved)
- Study eBinder: Select Perform Copy Certification from the Document Actions menu.
- Document Library: Select Perform Copy Certification from the All Actions menu.
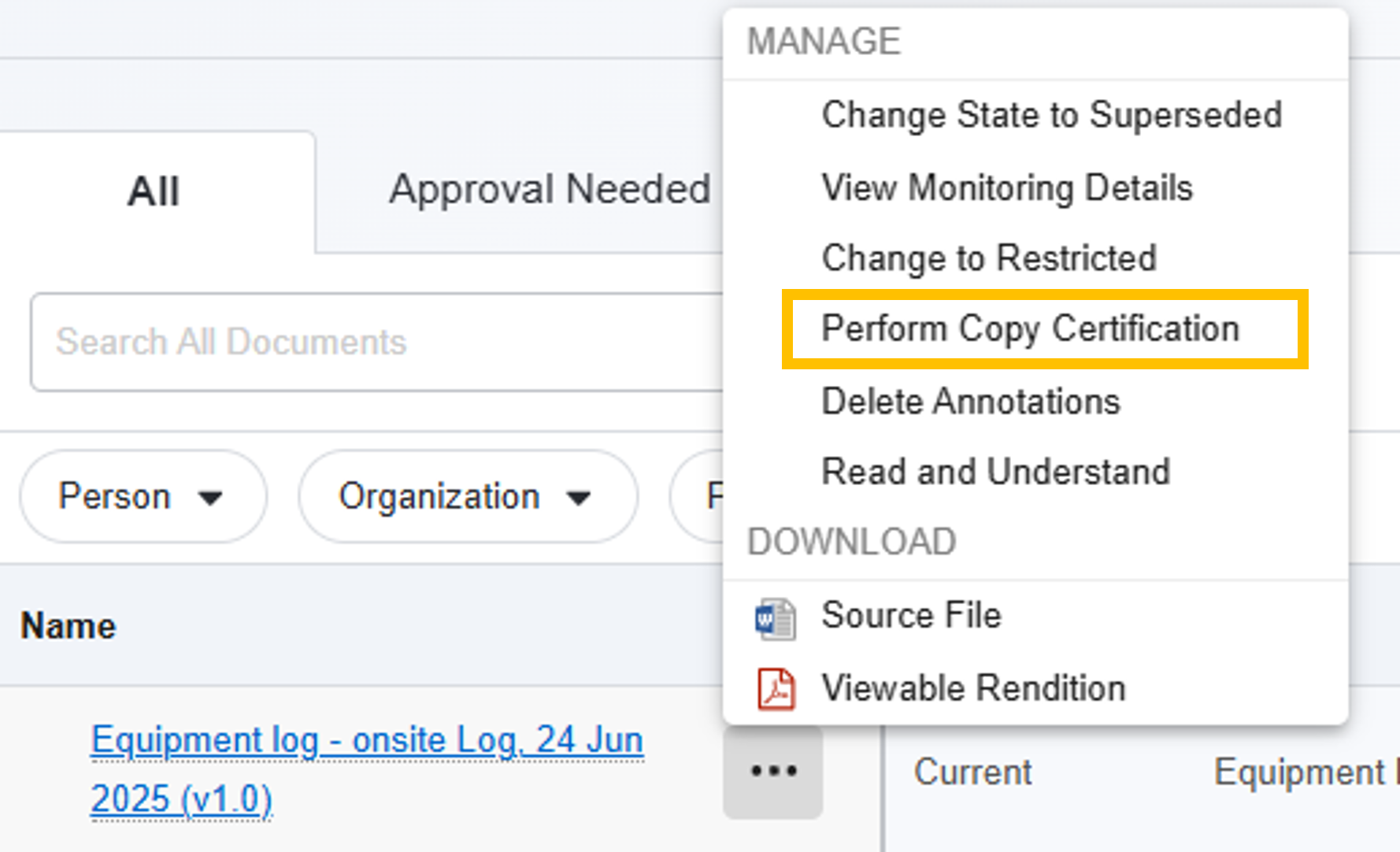
Identify a Certified Copy
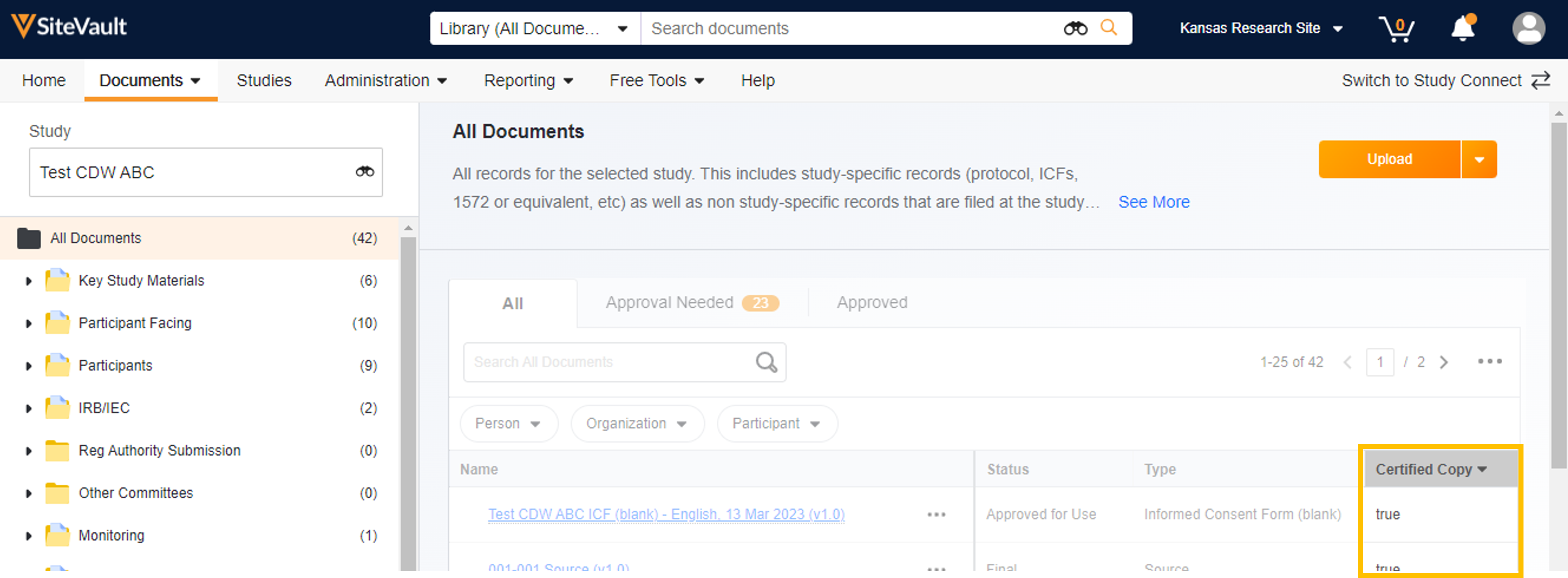
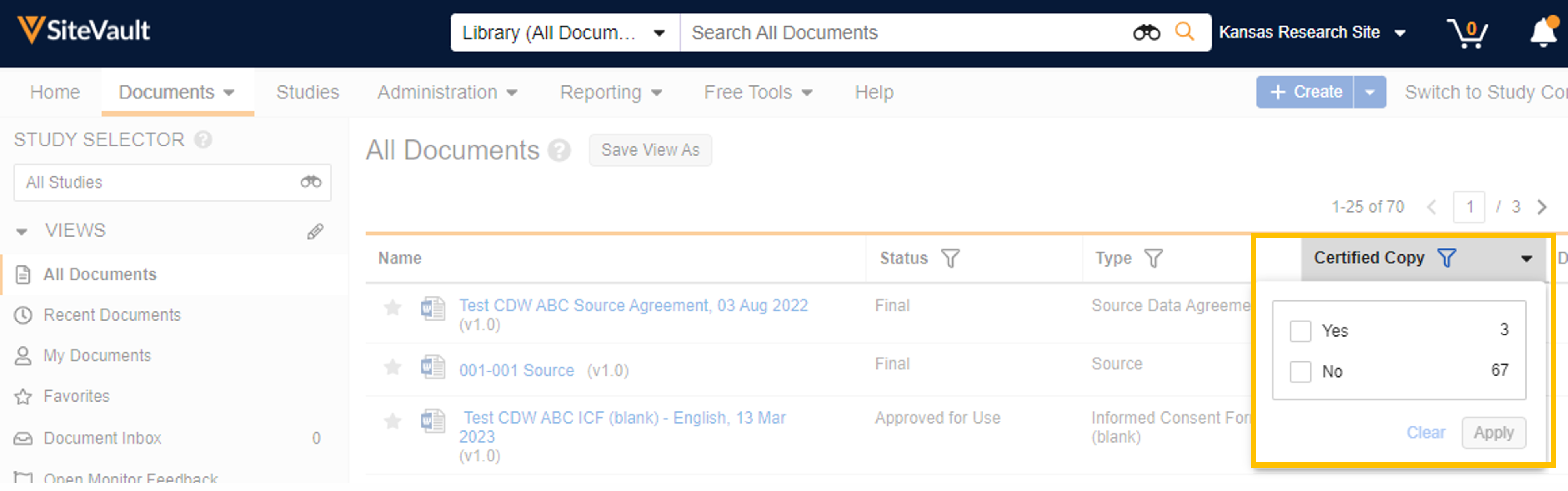
The Certified Copy field confirms whether the document has been certified as a copy.
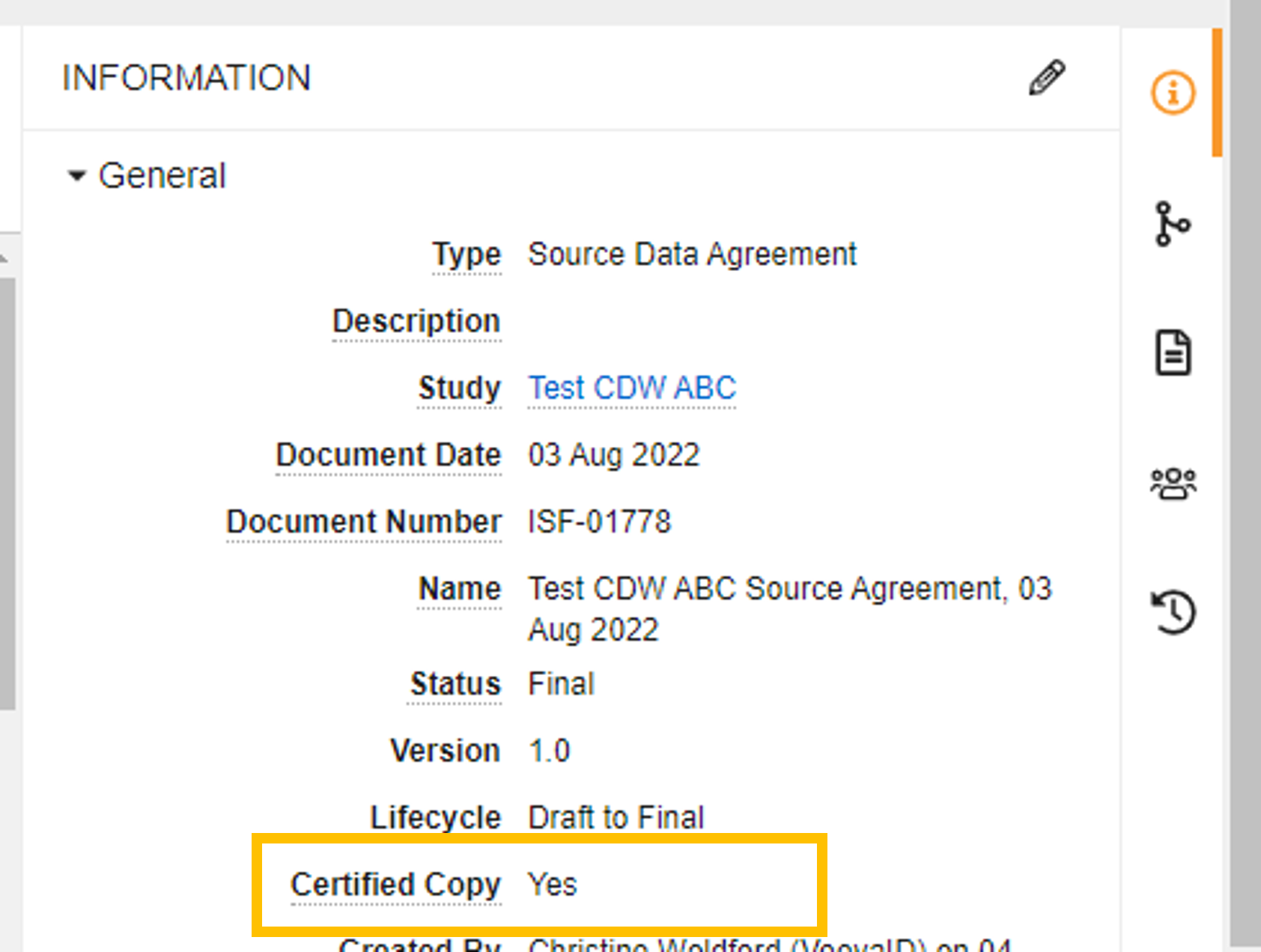
Locating Certified Copies
Use the Certified Copy document field to locate documents that are classified as a certified copy. Add the Certified Copy column to the Document Library and eBinder tables (All Actions Menu > Edit Columns). Certified copies will have a “Yes” or “True” in the Certified Copy field, depending on where you are searching.
Library View
Study eBinder View