About the Release
Important Dates
The release dates below are important for the Digital Trials Platform. See the About the 23R2 Release page in the Vault Release Notes for Vault release dates.
| Event | Date | Description |
|---|---|---|
| Pre-Release | July 17 | The release is deployed to pre-release Vaults and the MyVeeva for Patients pre-release environments. |
| Validation Package | July 17-August 11 |
The Digital Trials Platform validation package is available in VeevaDocs on the dates below. The Digital Trials Platform validation package includes validation documents for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO.
|
| General Release | August 11 | The release is deployed to all general release Vault PODs and MyVeeva for Patients general release environments. |
Release Information and Impacts
The release resources and information below are planned to be available on the dates in the Publication History column. See the About the 23R2 Release page in the Vault Release Notes for Vault resources and information.
| Resource | Publication History | Description |
|---|---|---|
| Digital Trials Platform 23R2 Release Impact Assessment | Updated August 1 |
The release impact assessment analyzes the regulatory, configuration, and user impacts of the MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO features in this release. |
| What's New | Available July 7 |
The What's New information provides detailed explanations of the new features for MyVeeva for Patients, SiteVault, Site Connect, Veeva eConsent, and Veeva ePRO. |
| Fixed Issues | Available August 11 |
The MyVeeva for Patients and SiteVault Fixed Issues lists at the bottom of this page list issues that affected previous versions or the pre-release and are fixed in this general release. |
| Known Issues | Available August 11 |
See the following pages for known issues that affect this general release or previous ones and aren't fixed yet: |
| Maintenance Releases | Both available with the first maintenance release |
See the following pages for fixed issues that affected the general release: |
| Digital Trials Platform Release Notes | See links |
See the following release notes for more information about new features across the Digital Trials Platform:
|
What’s New
MyVeeva for Patients
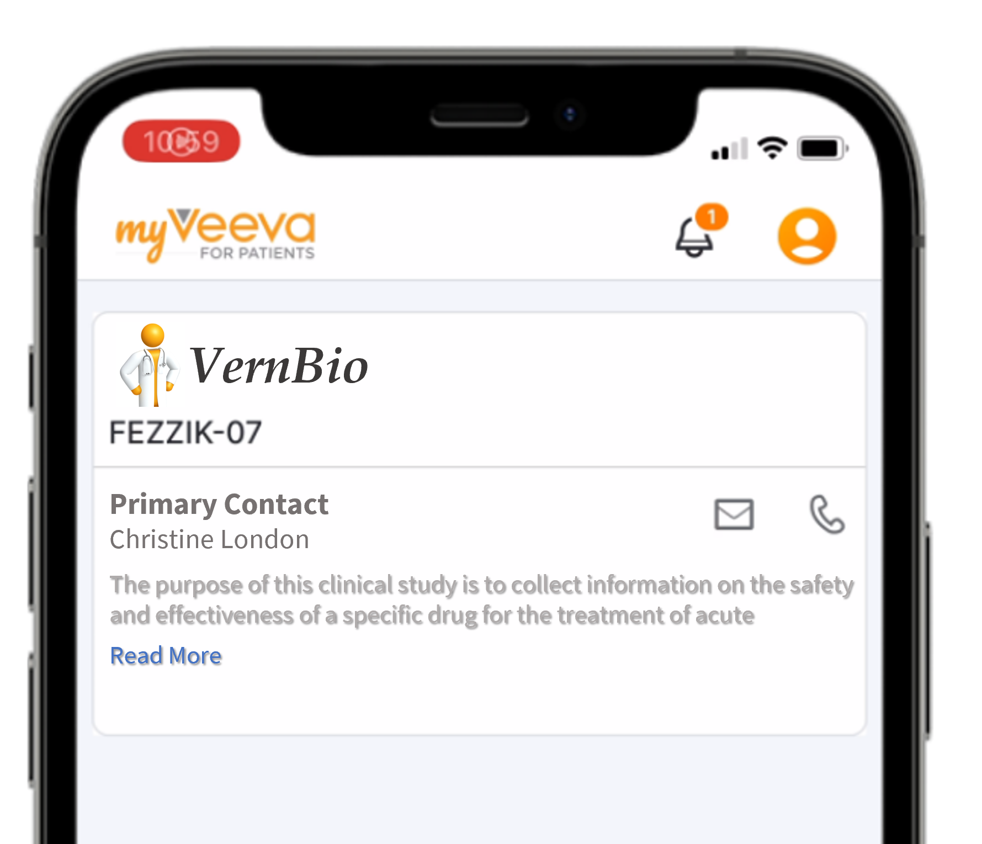
- Studies: Study Description, Image, and Contact Information
This feature allows MyVeeva users to reference sponsor- and site-provided study information on the Studies page in MyVeeva for Patients. Study builders can define study details and an image in MyVeeva Studio, and site users can designate a primary contact in Study Connect. The study details that sponsors provide are included in the study’s collection document.
-
PIN-Based MyVeeva Registration and Login
This feature allows MyVeeva users to register and log in to the Android and iOS apps with a self-created PIN. Site users can now generate a Device Registration Code (DRC) in Study Connect and provide it to a study participant or representative. When the participant or representative enters the DRC in the Android or iOS app, they’re prompted to create a device-specific PIN to log in and receive their consent forms, surveys, visits, and study documents during the study. This feature eliminates the need for participants and representatives to provide a valid phone number and email address to a site to register for MyVeeva for Patients.The following conditions apply to users who use a PIN:
- MyVeeva users who are already registered with a valid email address, password, and phone number are unaffected by this feature.
- Site users cannot generate a DRC for a participant or representative if an email address and phone number are present in their SiteVault record.
- MyVeeva users cannot use a PIN to log in to the web app.
- MyVeeva users who use a PIN to access their study materials are required to sign consent forms using a hand-drawn signature.
-
23R3 Mobile App Offline Enhancements
This feature provides MyVeeva users with an enhanced offline experience in the Android and iOS apps. While offline, users can now start, resume, and submit their assigned surveys as scheduled. When users interact with surveys while offline, their offline actions are now logged in audit trail data exports for sponsor and site users. The UI of the Tasks, Visits, and Documents pages are now refreshed to indicate to a user when they’re offline.The following conditions apply to offline users:
- Users may only remain offline in the mobile apps for thirty days before the system requires them to reconnect to a network and log in again.
- Users cannot view their shared study documents on the Documents page while offline.
- If a survey becomes overdue while a user is offline, the survey is removed from their Tasks page.
- Surveys that a user submits while offline are sent to the server once the user reconnects to a network.
-
23R2 Translations and Five New Patient Languages
This feature allows MyVeeva users to view application text, emails, notifications, text messages, and the terms of use and privacy policy in Spanish (US), English (UK), French (Canada), English (Canada), and Czech (Czechia). -
Push Notifications Displayed While App Is Open
This feature allows MyVeeva users to view push notifications while the mobile app is open on a device and to select the notification to be taken to the associated task, if defined.
SiteVault Release Highlights
SiteVault eReg
Study eBinder
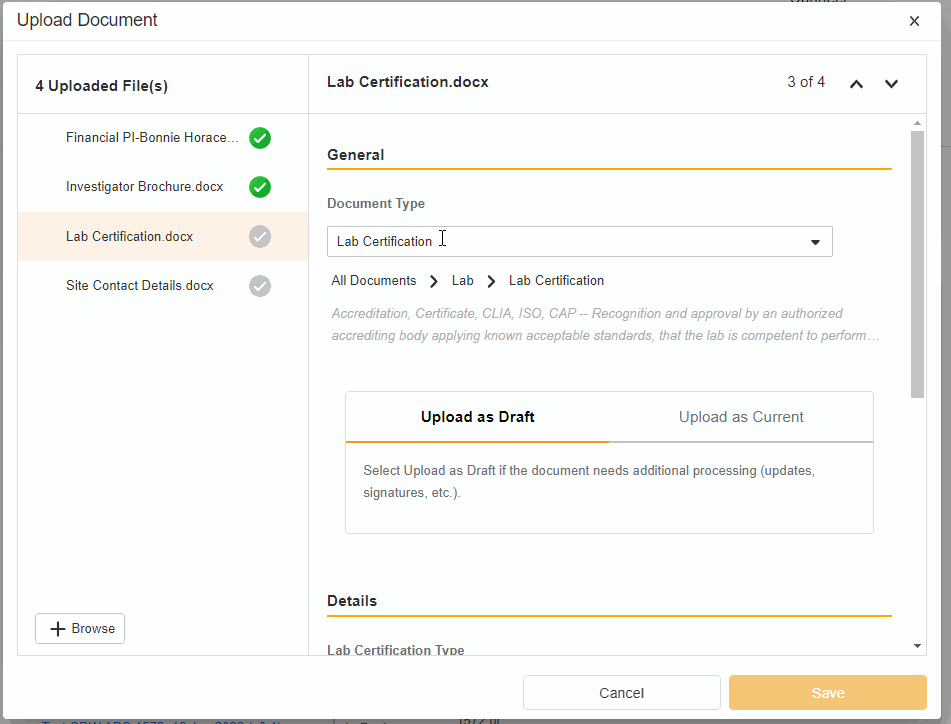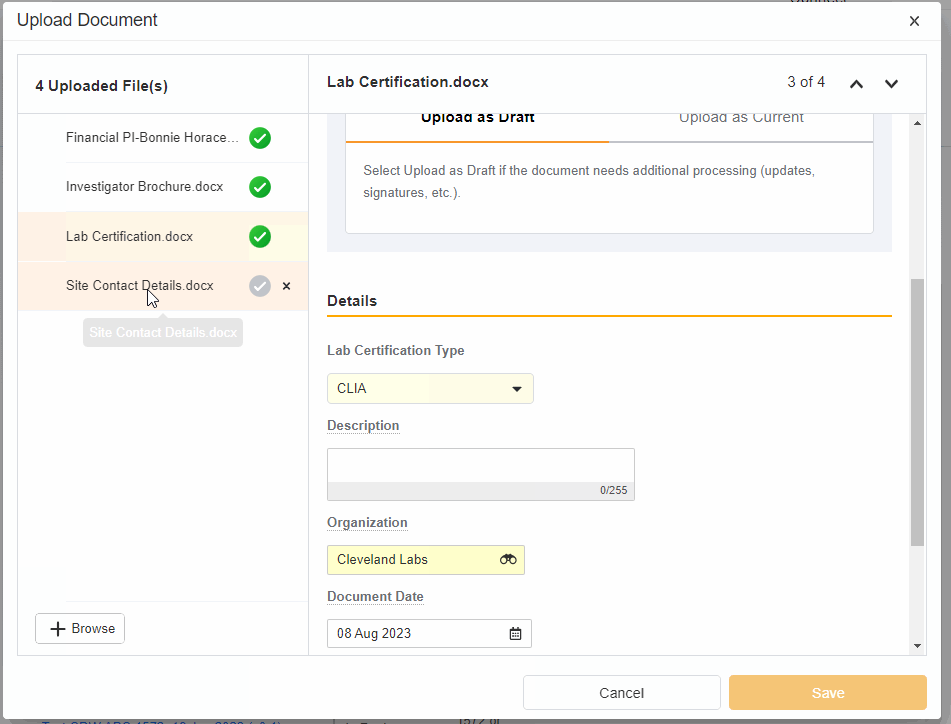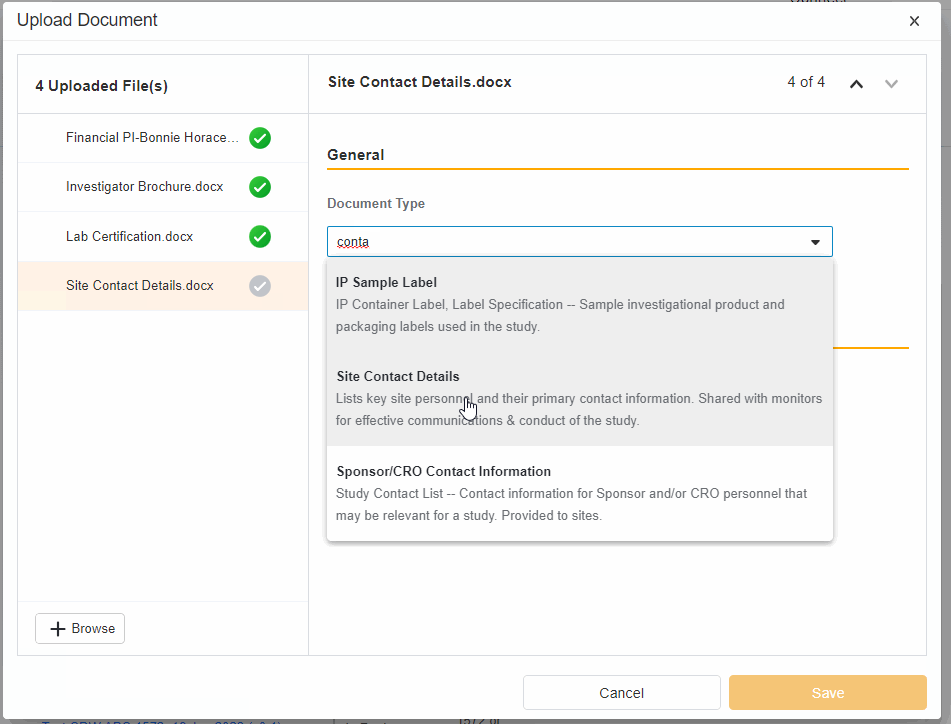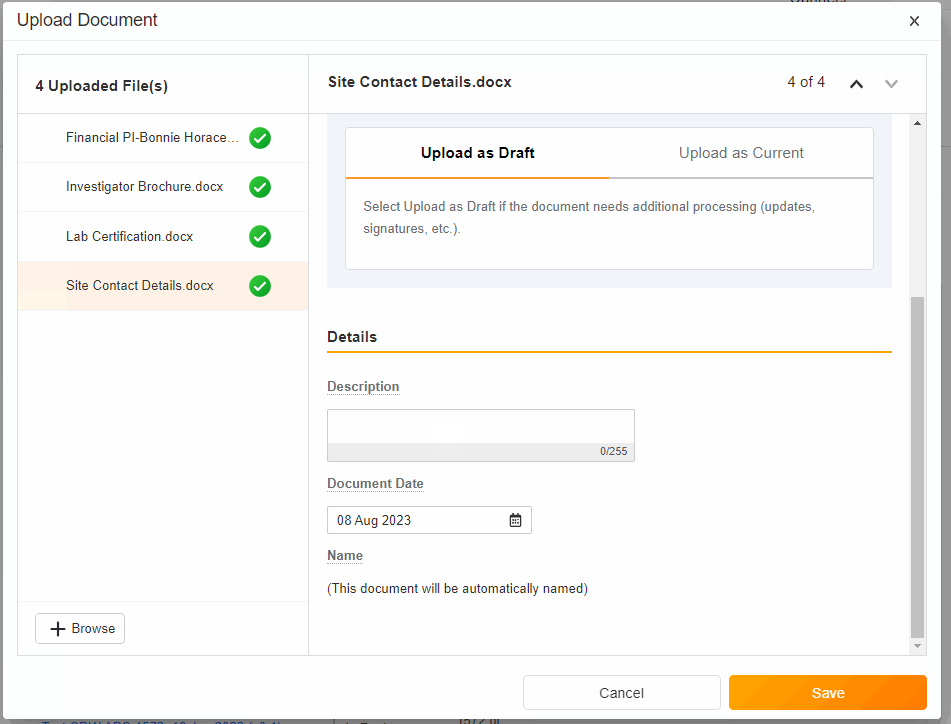
- Upload Multiples Files
This highly-requested feature expands the current eBinder upload functionality to include the ability to simultaneously upload multiple files with varying document types.
-
Bulk Source Upload
This feature shifts the bulk uploading of Source documents from the Source Upload tab to the Study eBinder. The helpful tools that allowed you to quickly populate fields in the Source Upload tab are also present in the eBinder Bulk Source Upload feature. Additionally, you have the ability to upload documents directly to their steady state. -
Create a Study, Organization, Product, or Participant from eBinder
This feature grants the ability to create a new Study, Organization, Product, or Participant directly from eBinder. Studies can be created from the Study Selector, while Organizations, Products, and Participants can be created during document upload.
Managing Users
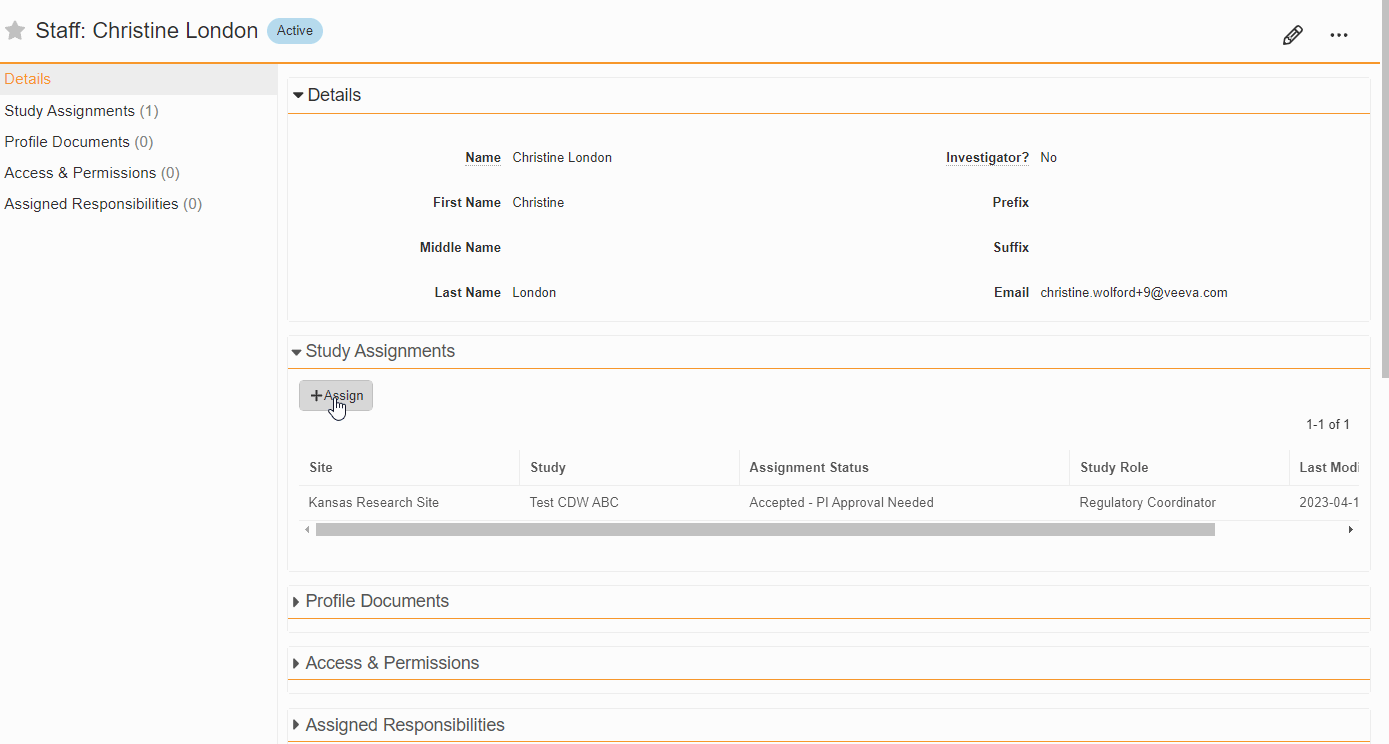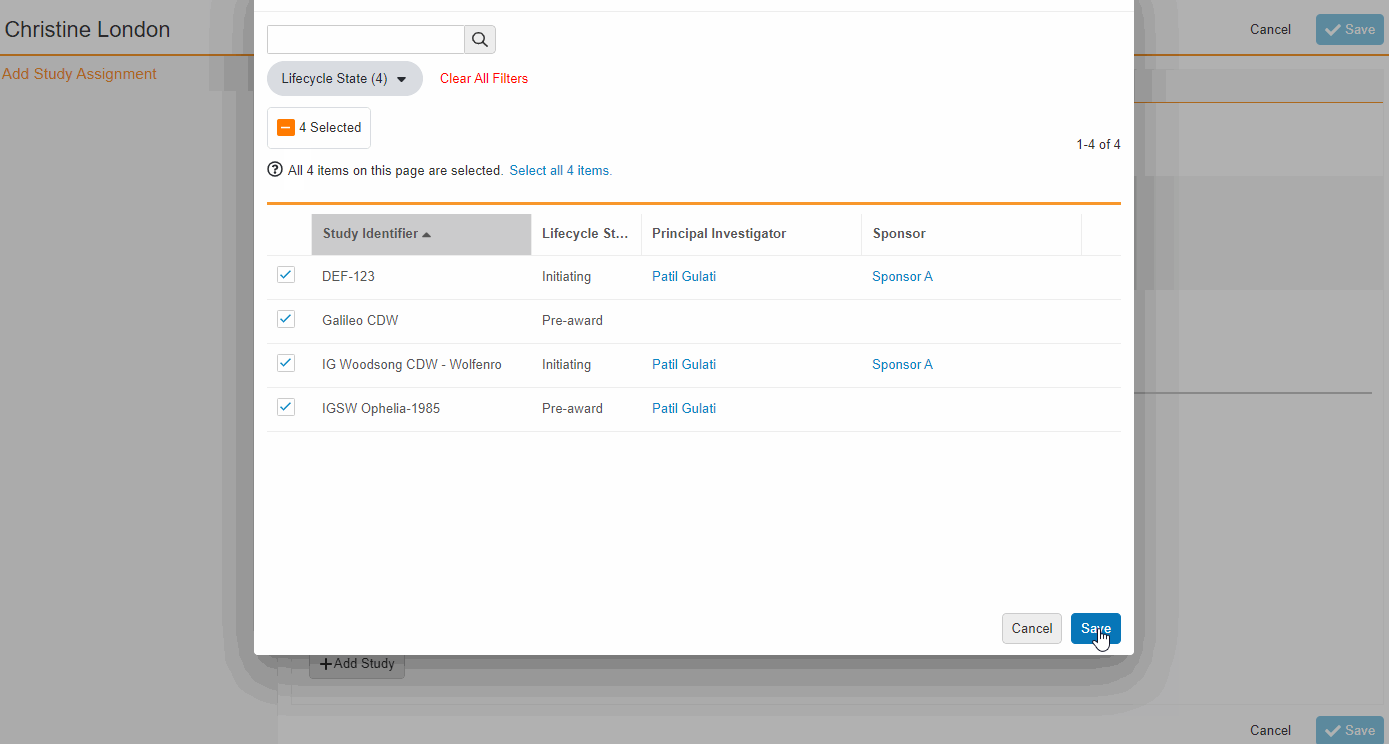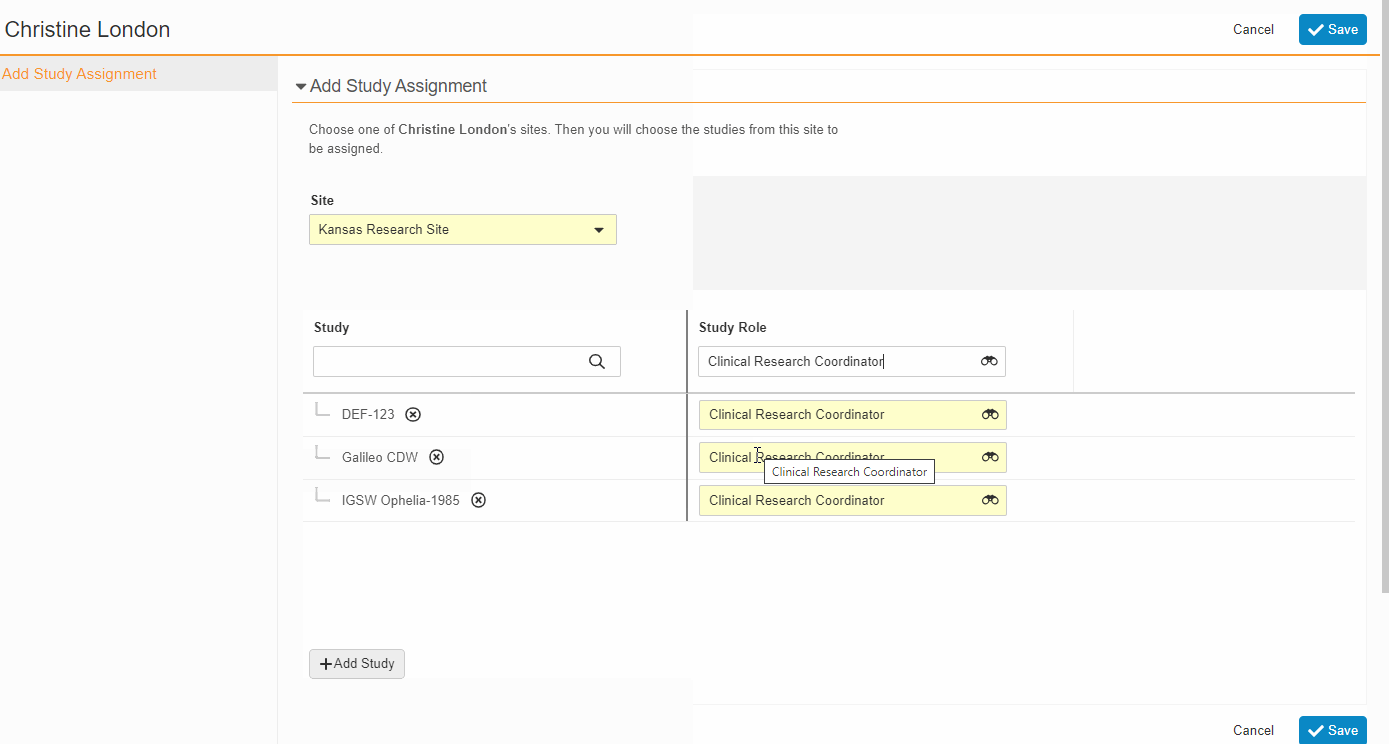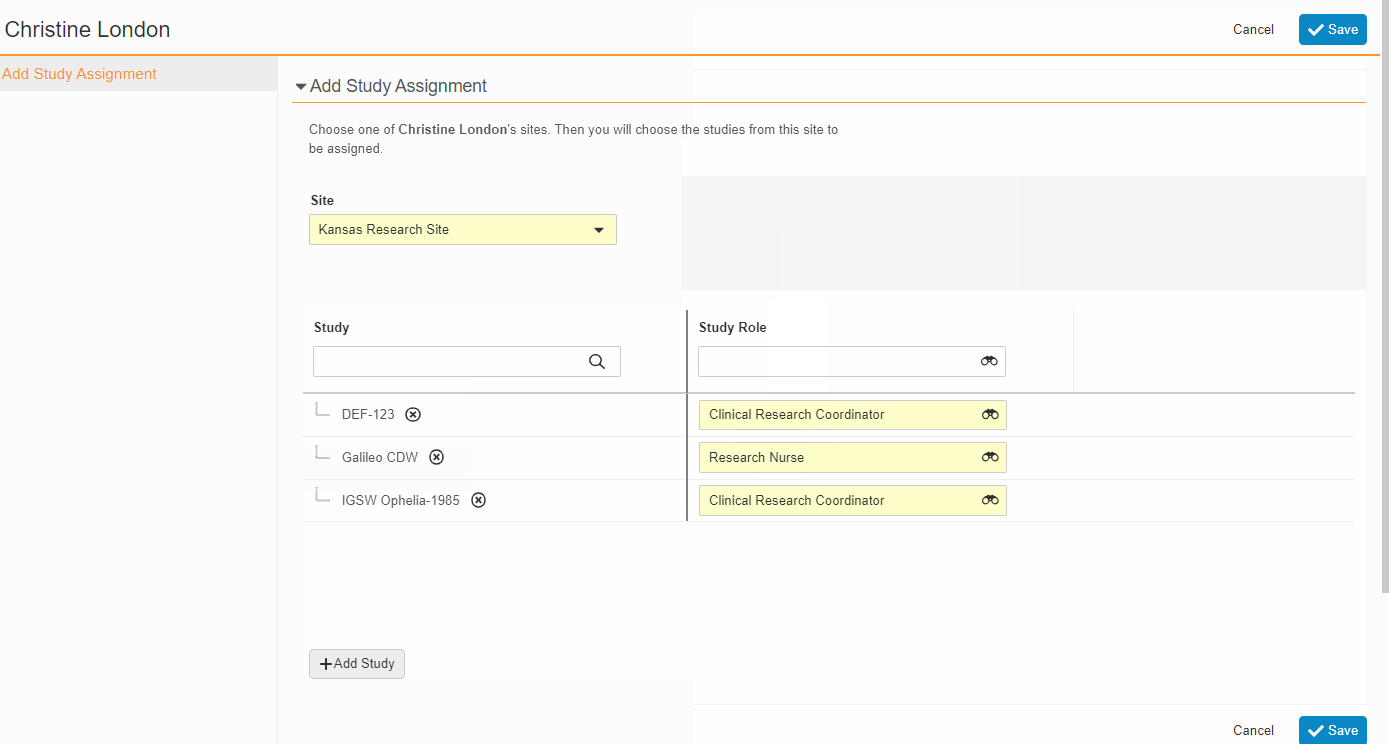
- Bulk Assign Staff to Studies
From the Staff Details Page (Administration > Staff), an Administrator can create Study Team Assignments for one or many studies. This enhancement includes the ability to apply a Study Role to all selected studies with a single click. The Staff Details Page contains a new section titled Study Assignments that displays assignments of all statuses, replacing the Active Study Assignments section.
-
Fully Inactivate a User and Their Study Assignments
This feature enables you to inactivate a user and their Study Assignments in a single step. This feature is available for managing both staff and external users. -
Staff Creation Updates
The Staff creation process is enhanced with an improved display of existing accounts utilizing the entered email address. -
Digital Delegation: Automatically Populate End Date when Inactivating Study Staff
For those using Digital Delegation, this feature expedites the process of inactivating a study staff member by adding their end date to all their delegations. When a Study Assignment state moves to Make Inactive, the user’s delegation End Date-Time fields inherit the assignment end date-time if they are empty or reflect an end date-time after the assignment end date-time. The delegation Start and End Date-Time fields remain editable while the assignment is in the Make Inactive state.
Managing Documents
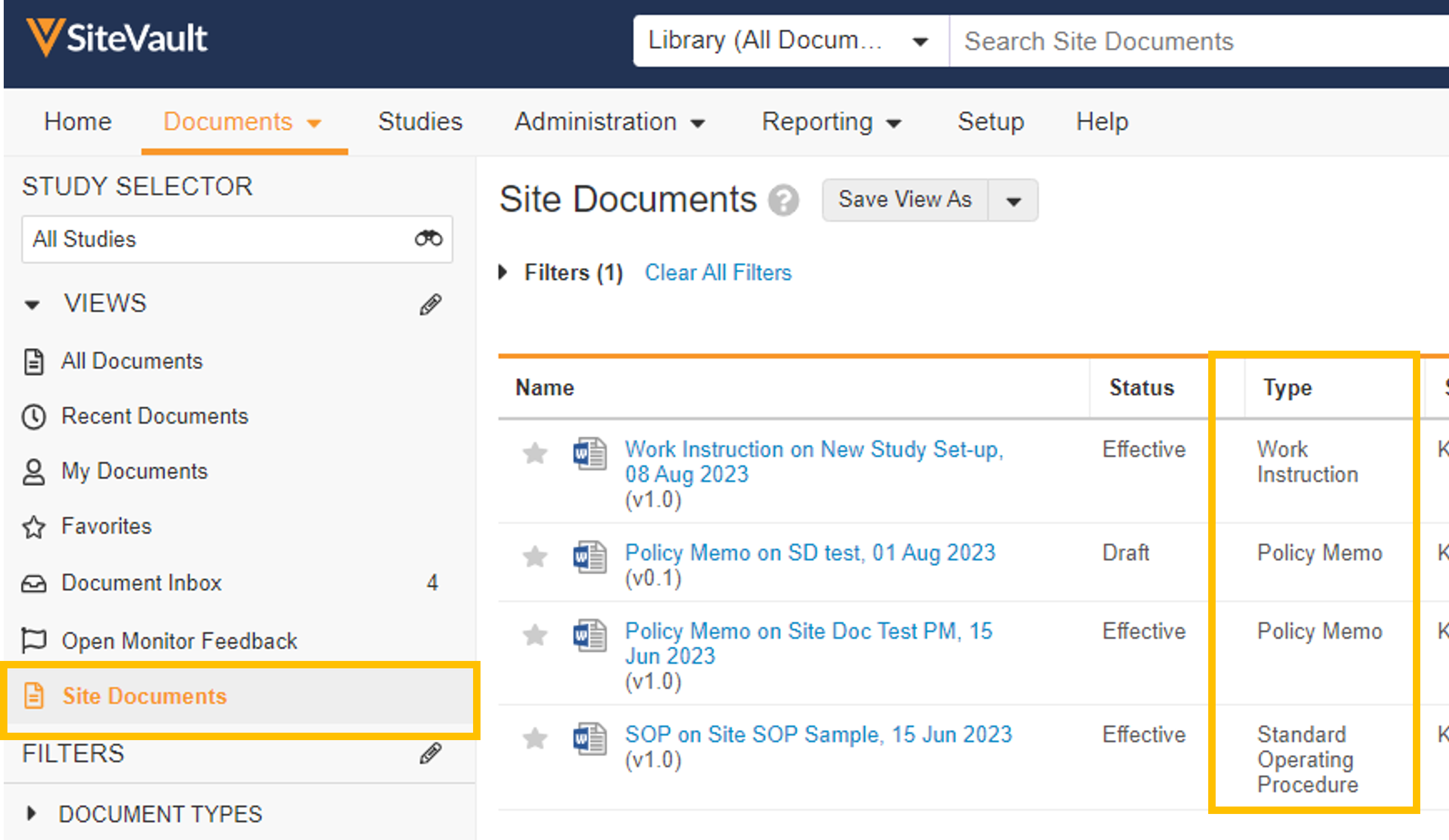
- Site Documents
You can use SiteVault to manage site-specific, non-study documents such as standard operating procedures (SOP), work instructions, and policy memos. These documents are stored in the Document Library, visible to Staff users once approved. Site Admins can create the documents and use workflows to send them for review, eSignature, and site staff training.
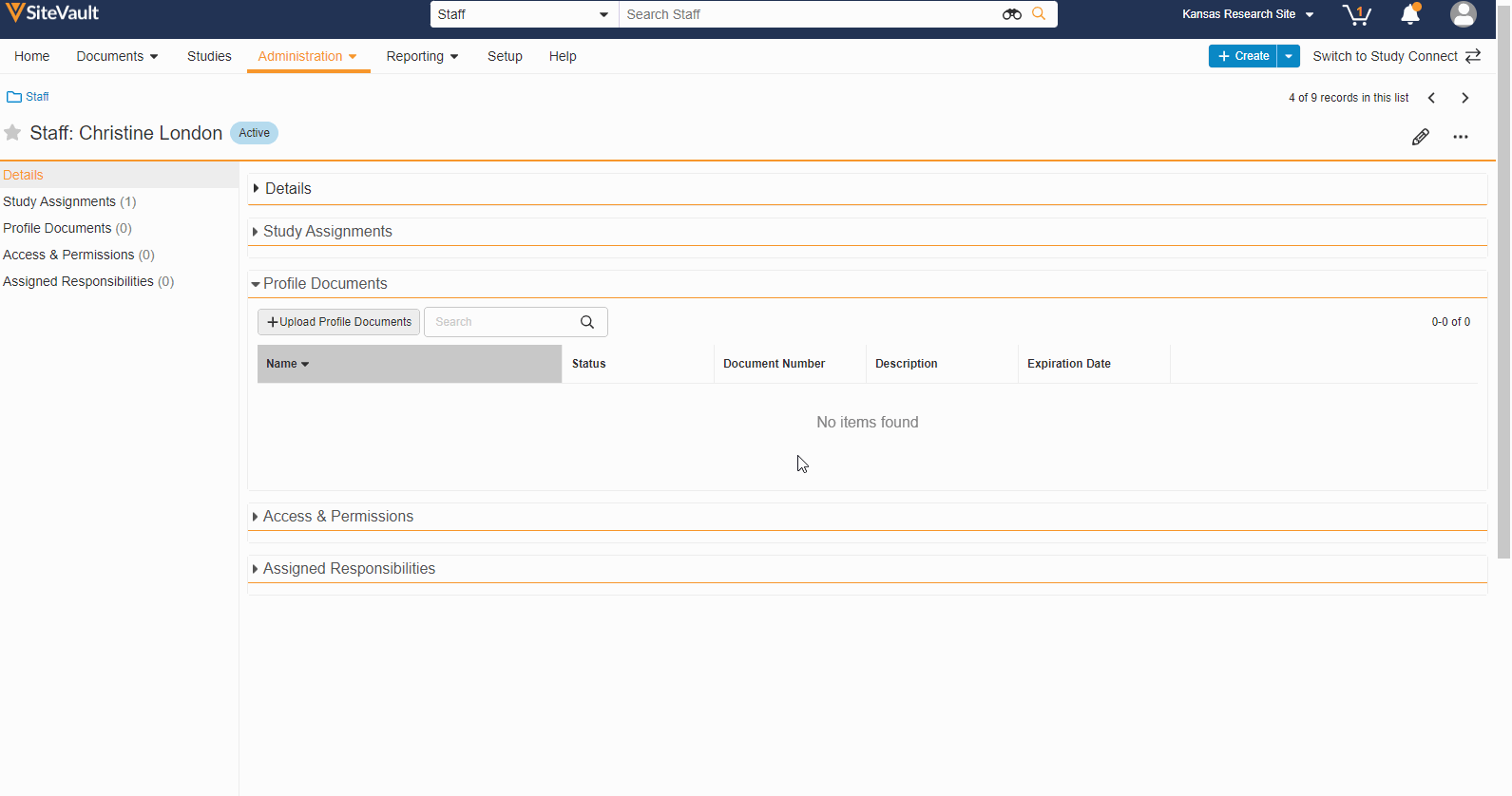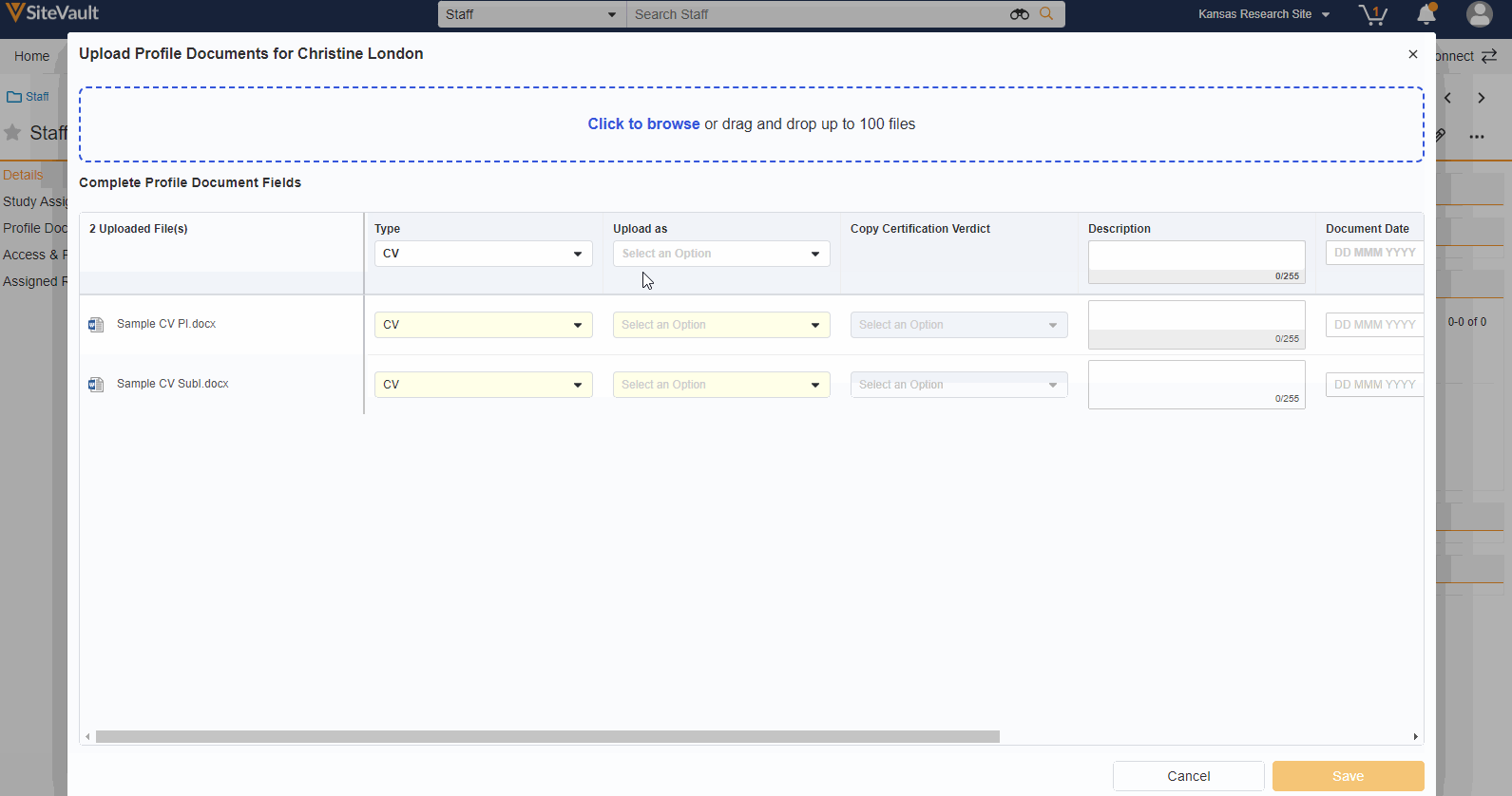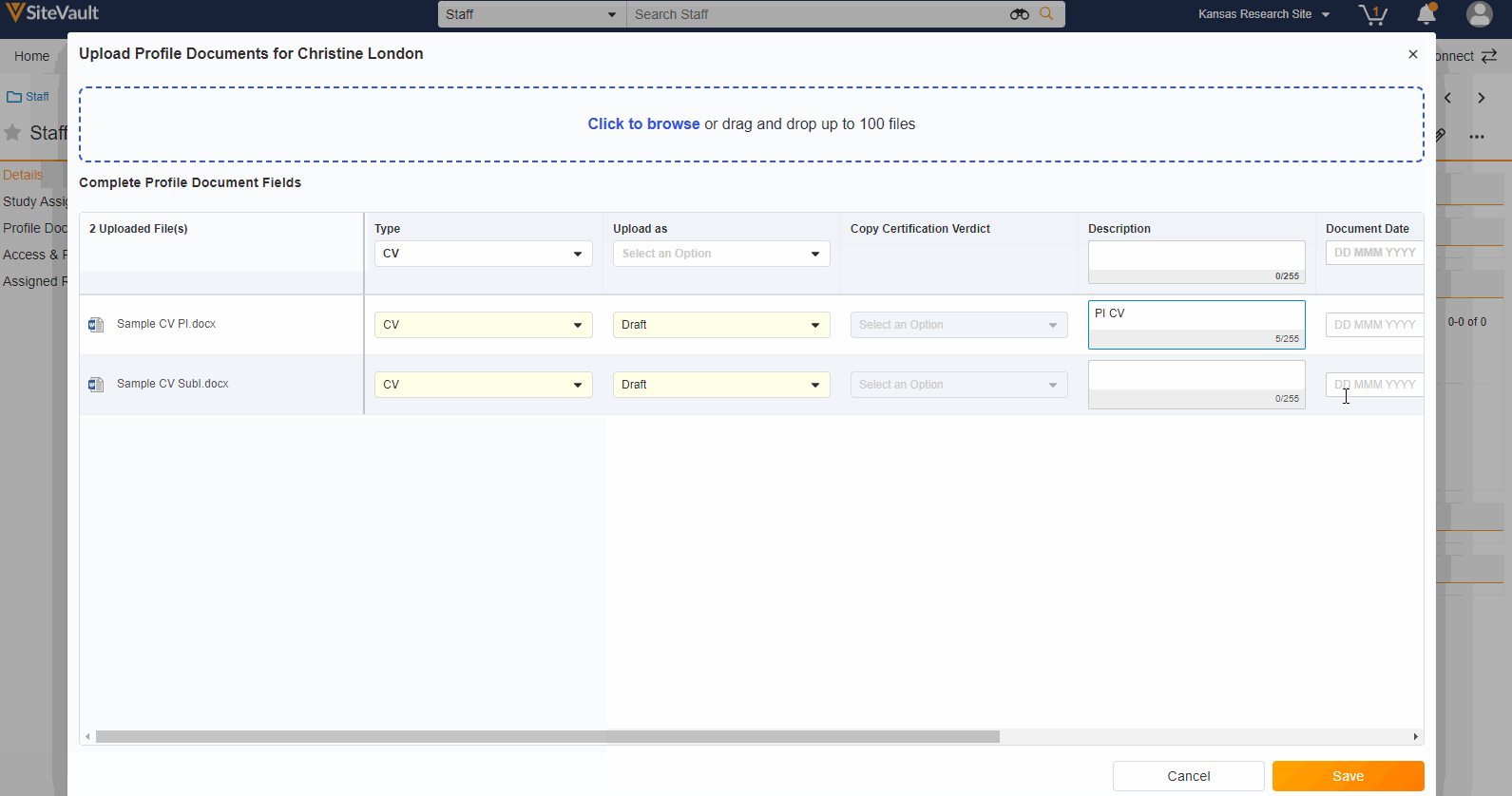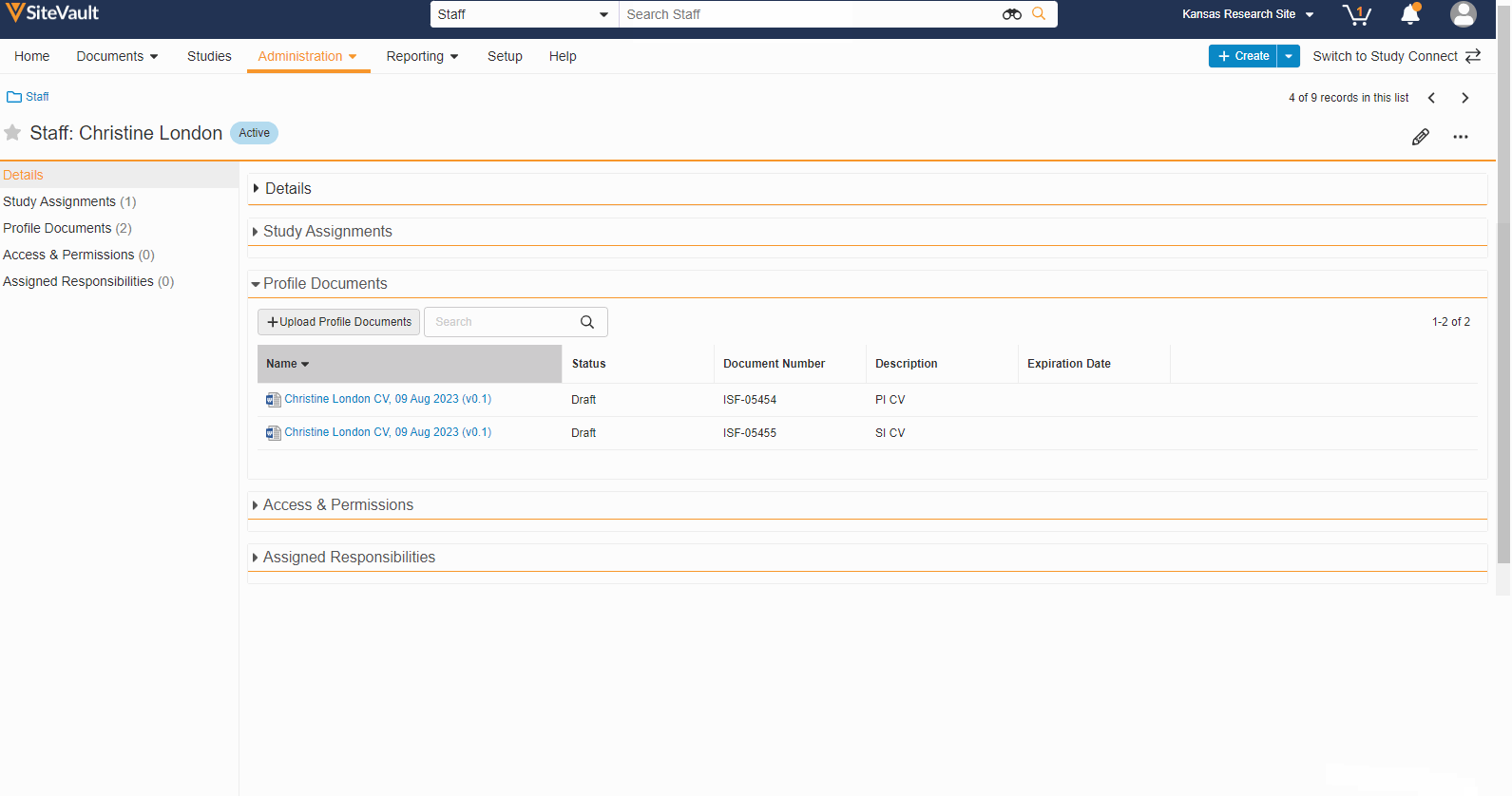
- Staff Profile Documents List
A Staff’s profile documents can be viewed and managed from the new Profile Documents section on the Staff Details page. From this section, Admins will also have the ability to upload documents (on the Person Profile lifecycle) directly to the Current state.
-
Workflow Instructions
When starting a document workflow, the workflow initiator can provide recipients with free-text instructions. These instructions will appear in the All and My Tasks notifications as well as on the document task ribbon.This feature is available on the following document workflows:
- Read and Understand
- Review Issues Found (Monitor)
- Send for eSignature
- Send for PI eSignature
- Send for Review
-
Update Document Reuse Logic
When activating a new Staff or Organization on a study, the most recent steady state version and any subsequent draft versions of profile documents are filed to the study. Previously, only the most recent version was filed to the study, regardless of document state. -
Participant ID Code List Document Type Update
The Participant ID Code List document type is now on the Draft to Current - Source document lifecycle. Files associated with this document type cannot be downloaded by external users.
Additional New Features or Enhancements
-
SiteVault Inviter
With this feature, Sponsors/CROs can assist sites with signing up for SiteVault, initiating the process from within Clinical Operations. Clinical Vault users can enter a site staff email address into the Inviter to identify if a site is already using SiteVault. If a site is using SiteVault, the USN is visible in the Clinical Vault. If a site is not using SiteVault, the site is invited to sign-up and the USN is available in the Clinical Vault upon sign-up completion. -
Patient Navigation Update
Staff users can continue to view, create, and edit Patient records by navigating through a study (Participant); however, the Administration > Patients tab will no longer be visible. -
Additional Languages with Locales
This feature adds the following four language locales to the list of available languages for document and patient records in SiteVault.- Czech Czechia (cs-CZ)
- Dutch Belgium (nl-BE)
- English Canada (en-CA)
- English Ireland (en-IE)
- French Belgium (fr-BE)
- French Canada (fr-CA)
- French Swiss (fr-CH)
- German Austria (de-AT)
- German Swiss (de-CH)
- Italian Swiss (it-CH)
- Spanish US (es-US)
For more information, see Supported Languages.
SiteVault Study Connect
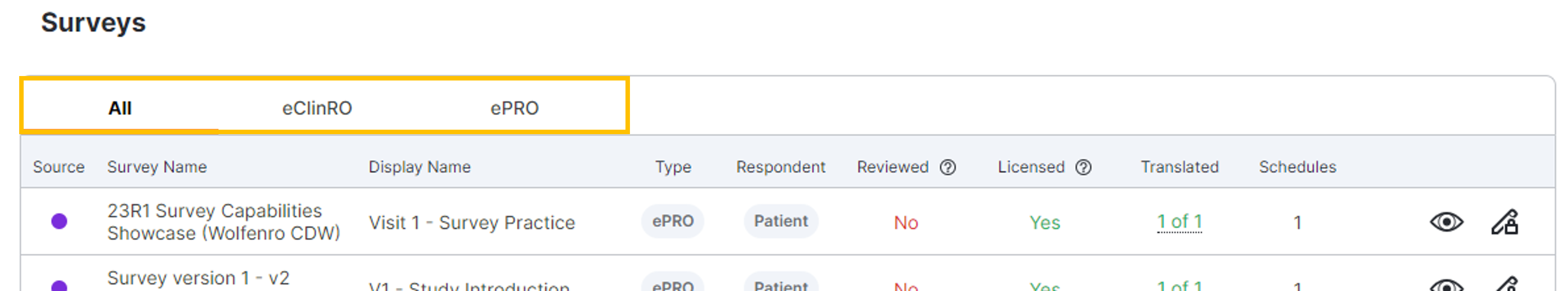
ePRO
-
eClinRO in Study Connect
This feature expands the current ePRO functionality to include other types of Electronic Clinical Outcome Assessments (eCOA) and enhances the user experience when accessing, viewing, and interpreting surveys in Study Connect.Site users can:
- Manage and submit electronic clinician-reported outcome (eClinRO) surveys in Study Connect
- View completed ePRO and eClinRO surveys in SiteVault
- View eClinRO-related events in audit trail data exports
From a participant event in the participant record in SiteVault, site users can view a survey list related to that participant event and complete and submit the surveys. When a site user begins an eClinRO survey, it’s locked and made viewable in read-only format for other site users until the responding user submits, becomes inactive, or exits the survey. Site users can view completed eClinRO surveys, and audit trail data exports now include eClinRO-related events.
Studies using only eClinRO surveys are not required to approve the collection document prior to enabling ePRO for the study. When upversioned collections include both ePRO and eClinRO surveys but only modify the eClinROs, the collection version for participants is automatically updated.

-
eClinRO and ePRO - Participant Event Enhancements
This feature provides site users with an enhanced participant event list in Study Connect. They can drill into a participant event to view all related ePRO and eClinRO surveys. Each survey is identifiable by its status, and the user can begin new or view completed surveys. Additionally, users can toggle the list to view only the available surveys. When entering eClinRO surveys or viewing completed eClinRO or ePRO surveys, the site user can switch between the languages that the survey supports. -
Survey Compliance Warnings
This feature allows site users to view survey compliance warnings in Study Connect. When participants have important surveys coming due or have missed surveys in the last seven days, a new widget on the Surveys Overview page displays detailed information about compliance for those participants. From the widget, site users can access participant contact information and add notes. Compliance warnings for surveys that are coming due soon are derived from the survey’s “Survey Due” site notification configuration, which is defined by a study builder. -
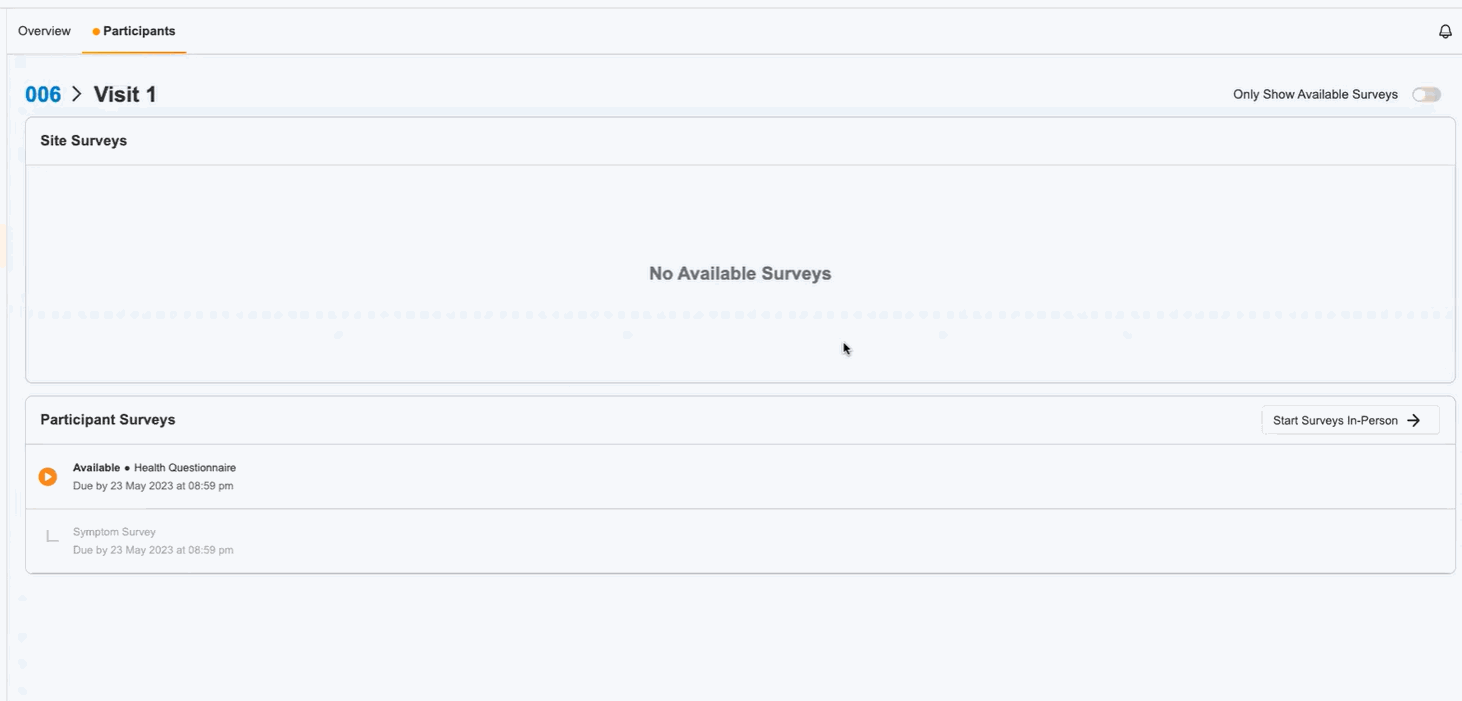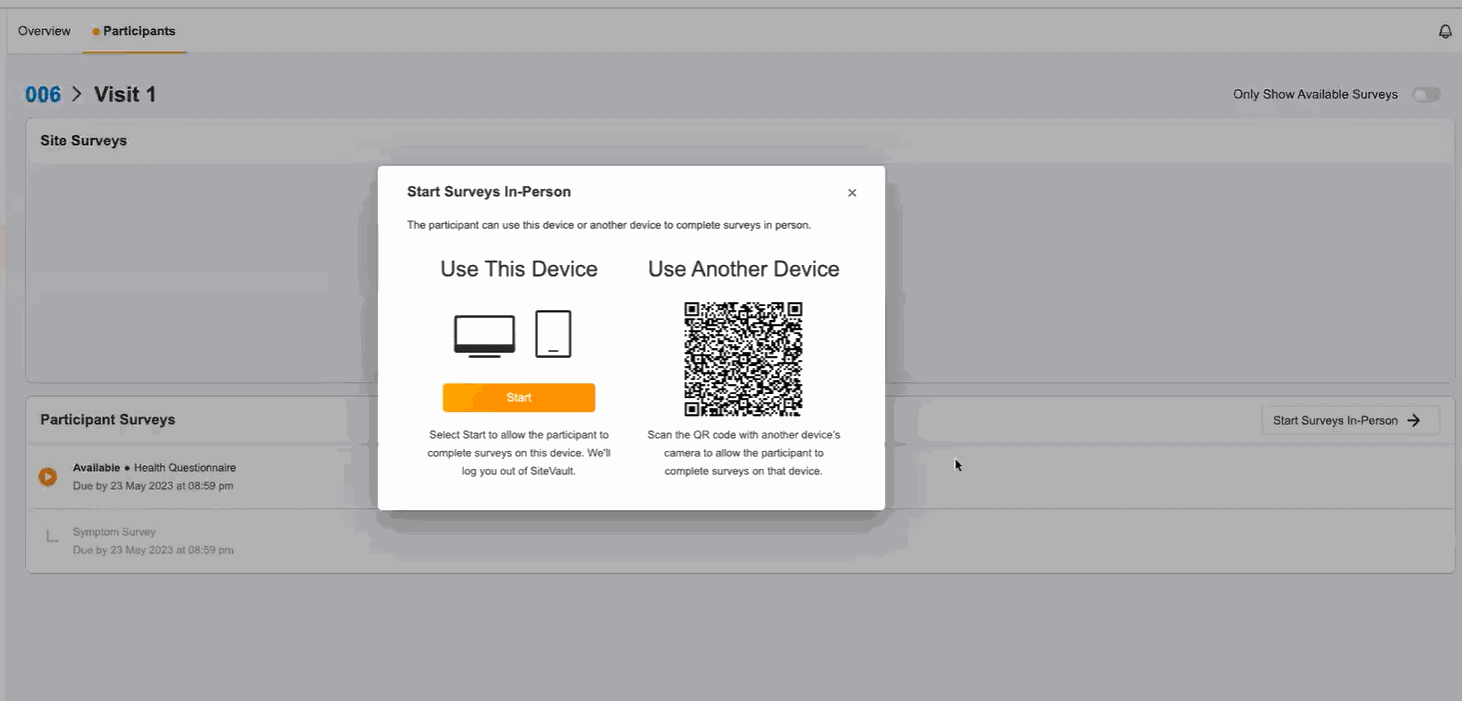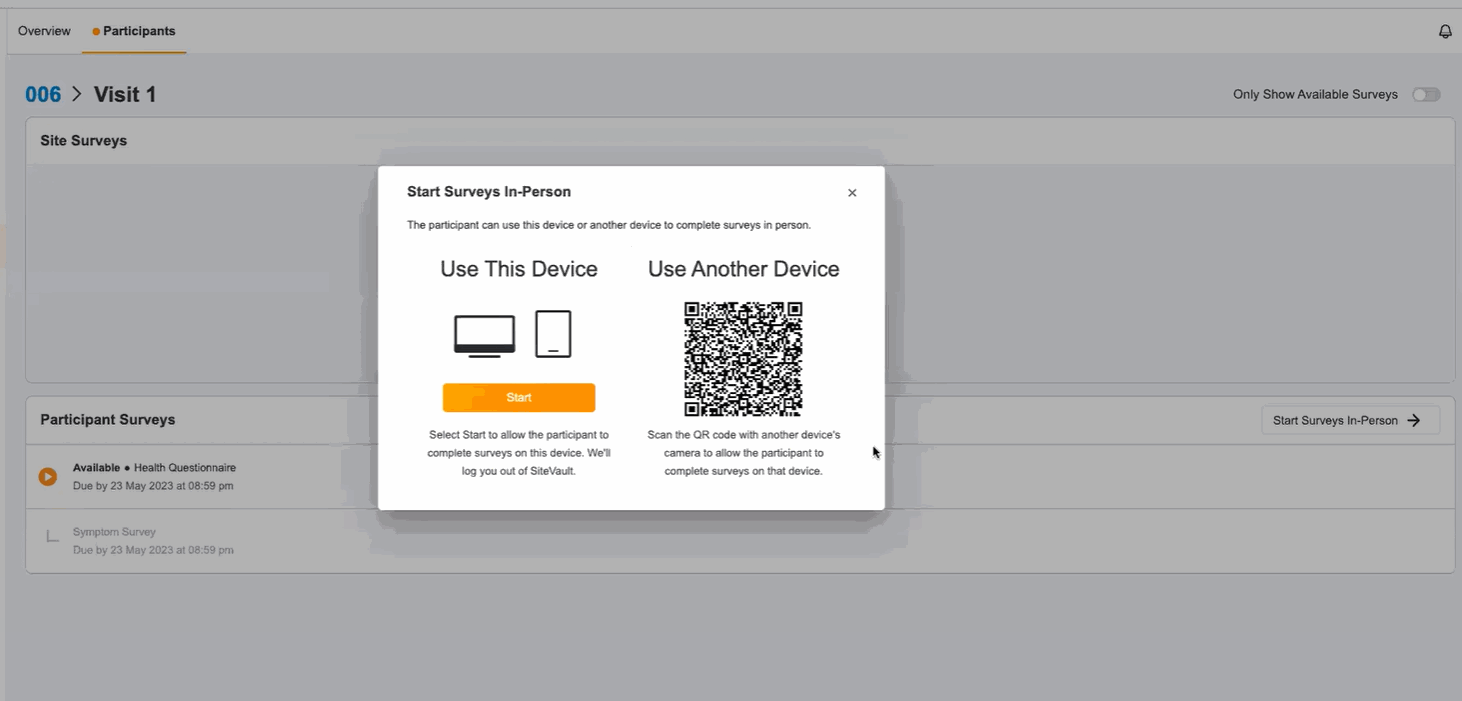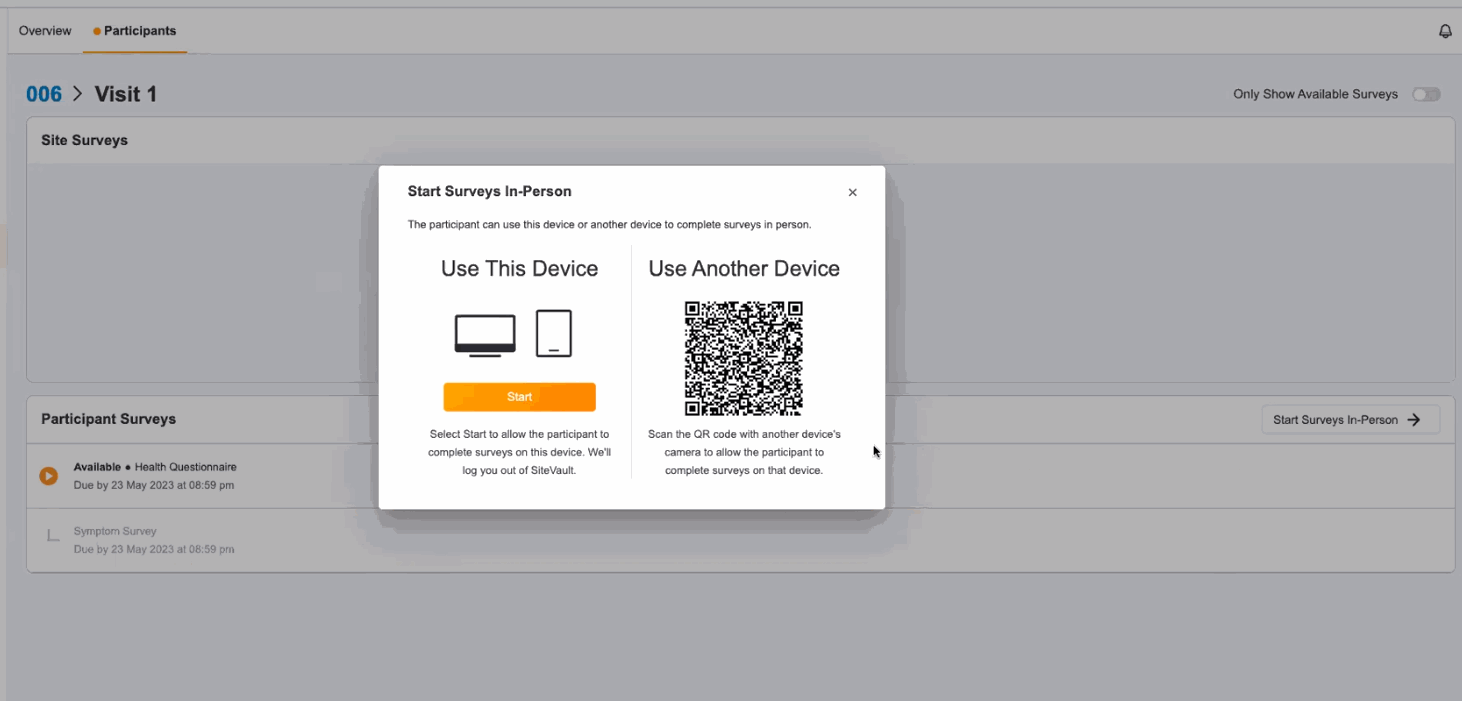
In-Person ePRO Survey Completion
This feature provides participants with the option of performing ePRO activity while visiting a site, without requiring a MyVeeva for Patients account. You can start a participant’s in-person session from the participant event list in Study Connect. Participants can either complete their surveys on your site’s device or on a separate device. During an in-person survey session, MyVeeva users can complete any study surveys that are currently available to them.

-
eClinRO and ePRO - Data Export Enhancements
This feature enhances survey, adherence, and audit trail data exports for sponsor and site users by including new data related to eClinRO. New SITE STAFF values can populate in Completed By columns related to eClinRO surveys. New ePRO Survey and eClinRO Survey values can populate in Survey Type columns. These additional values may impact any external post-export data loads that users have set up for data analysis. -
My Veeva for Patients PIN Access
This feature allows ePRO enablement for participants who prefer or cannot provide an email address or phone number. You can generate a Device Registration Code in Study Connect and provide it to a study participant or representative. When the participant or representative enters the DRC in the Android or iOS app, they’re prompted to create a device-specific PIN to log in and receive their consent forms, surveys, visits, and study documents during the study.

Document Exchange
- eReg Document Number Displayed in Study Connect
The eReg Document Number is visible from all Document Exchange Document tabs and Study Connect documents. You will now also be able to search for eReg document numbers within Study Connect.
- Document Exchange Document Tabs: Filed eReg Number column
- Study Connect Documents: eReg Document Number document field located in the Sponsor/CRO Information section
- Sponsor Name on Document Exchange Notifications
This feature adds the name of the sponsor to the subject line of Document Exchange emails and to the notifications in the Study Connect notifications panel.
Additional New Features or Enhancements
- My Veeva for Patients PIN Access
From eConsent or ePRO Participant pages, you can generate a Device Registration Code in Study Connect and provide it to a study participant or representative when a phone number or email address is not available. When the participant or representative enters the DRC in the Android or iOS app, they’re prompted to create a device-specific PIN to log in and receive their consent forms, surveys, visits, and study documents during the study.

-
Additional Details in Study Connect Action Needed Notifications
Study Connect weekly action needed notifications now include task details and due dates so you can more easily identify what action is needed. -
MyVeeva Study Home: Provide Site Contact Information
This feature enables sites to designate a primary contact that can be shared with participants in the MyVeeva for Patients application.

- Search Studies in Study Connect
With this feature, you can now search for studies in the Studies tab.

SiteVault Data Model Changes
With every release, we update the SiteVault data model to better support evolving needs and new feature functionality. See 23R2 Data Model Changes for more information.
Veeva eConsent
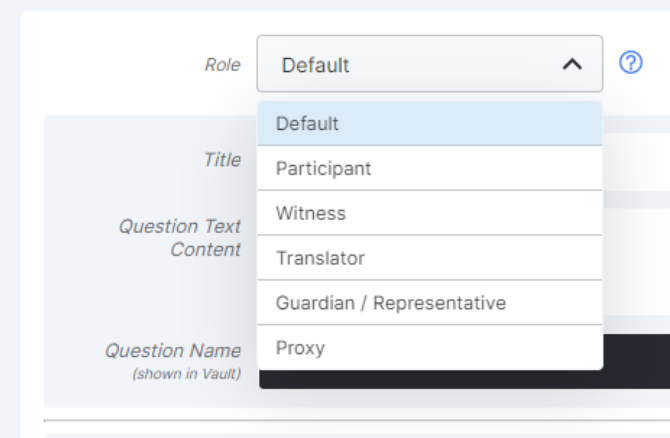
- Role-Specific eConsent Form Questions
This feature allows sponsor and site users to assign eConsent question blocks to specific signatory roles. Multiple roles can answer questions in a single eConsent, and other roles don’t need to respond. Users can now also preview an eConsent form as a specific role by creating a preview link of the form and specifying the role in the link URL.
- Server-Generated eConsent HTML
This feature enhances eConsent performance by having the server generate the HTML and PDF renditions of forms in the eConsent editor and the eConsent viewer. Previously, HTML and .PDF renditions of eConsent forms were generated by the Android, iOS, and web app clients. This feature corrects issues that users may have encountered with Word-based eConsent forms if they did not edit the form in the editor prior to approving it for use.
For more information, see Editing eConsent Forms in Clinical Vault Help.
Veeva ePRO
ePRO Module Enhancements
-
Change ePRO Module to MyVeeva Studio
Veeva ePRO technology has continually evolved since 22R2, and we’ve expanded its capabilities with additions such as electronic clinician-reported outcomes (eClinROs) and visit management. To deliver a consistent experience to sponsor and site users, we’ve made the following changes to the names and locations of these features:- The ePRO Module is now called the MyVeeva Studio.
- References to ePRO have been removed from the sponsor and site experiences in the Clinical Operations Vault and SiteVault.
- ePRO collections and ePRO collection documents are now called collections and collection documents.
- Audit activities for sponsors and site users no longer display the word “ePRO”.
- Participants using the MyVeeva for Patients application are not affected by these changes.
For more information, see Using MyVeeva Studio in Clinical Vault Help.
-
eClinRO - Sponsor Configuration and Actions
This feature allows study builders to configure electronic clinician-reported outcome (eClinRO) surveys in MyVeeva Studio for site users to complete in SiteVault. Study builders can create surveys using JSON or import surveys from the library. Users can define which study languages are patient- or site-facing and define if eClinRO surveys should use patient languages instead of site languagesFor more information, see Configuring Surveys in Clinical Vault Help.
-
eClinRO and ePRO - Data Export Enhancements
This feature enhances survey, adherence, and audit trail data exports for sponsor and site users by including new data related to eClinRO. New SITE STAFF values can populate in Completed By columns related to eClinRO surveys. New ePRO Survey and eClinRO Survey values can populate in Survey Type columns. These additional values may impact any external post-export data loads that users have set up for data analysis.For more information, see Exporting Survey, Adherence, and Audit Trail Data in Clinical Vault Help.
-
FTP Enhancements: One-Time Transfers, Custom File Names, and Inactivate Connection
This feature enhances FTP survey data transfers for sponsors. All studies with an established FTP connection (either through CDB or an external FTP) can now run one-time transfers. Users with external FTP connections can choose which files are included in daily transfers and customize the file names. Additionally, all users can now inactivate FTP connections.For more information, see Exporting Survey, Adherence, and Audit Trail Data in Clinical Vault Help.
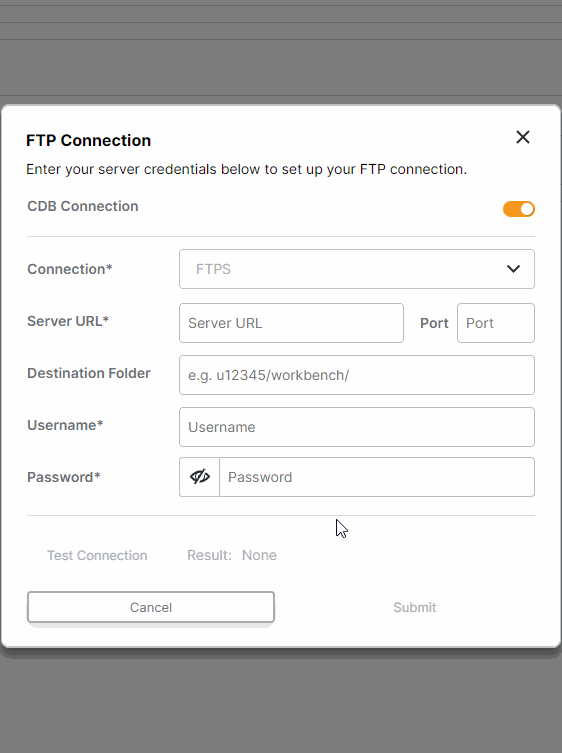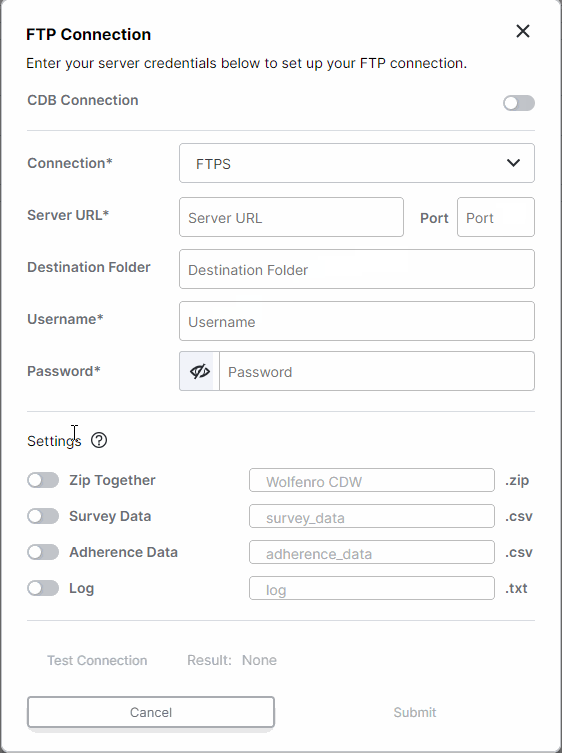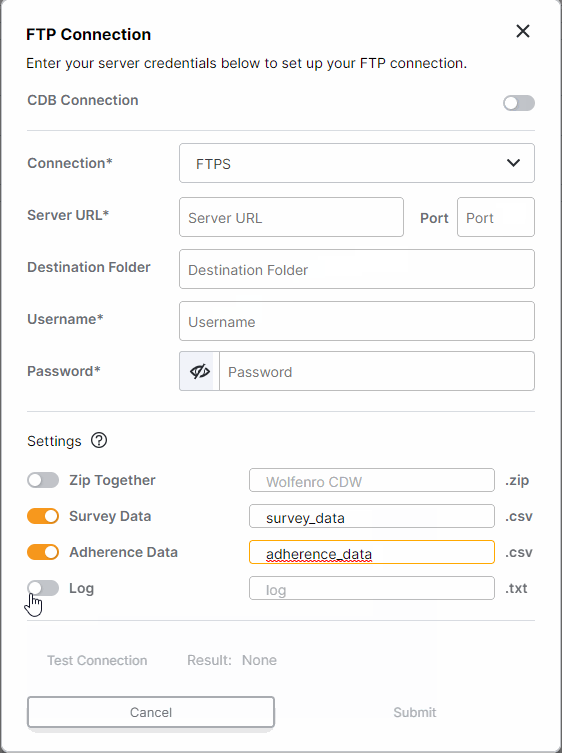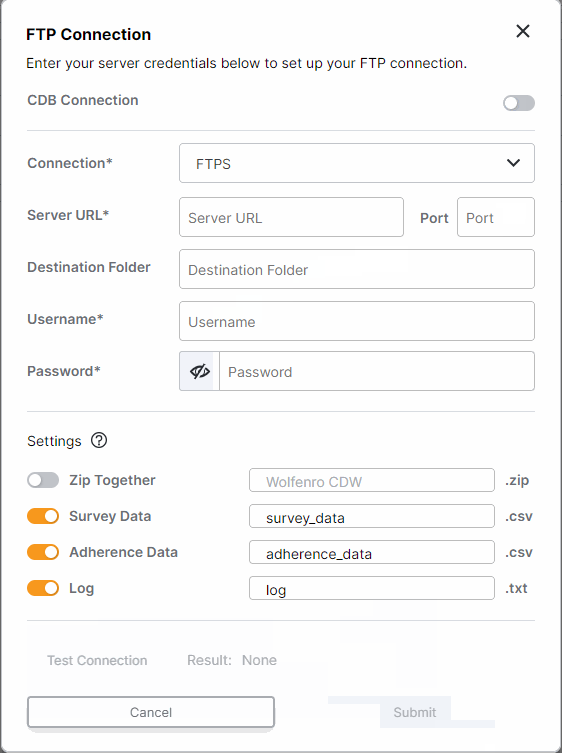
-
Survey Library Search
This feature allows study builders to search for surveys by name when adding a survey to their collection from the library.For more information, see Using Survey Libraries in Clinical Vault Help.
-
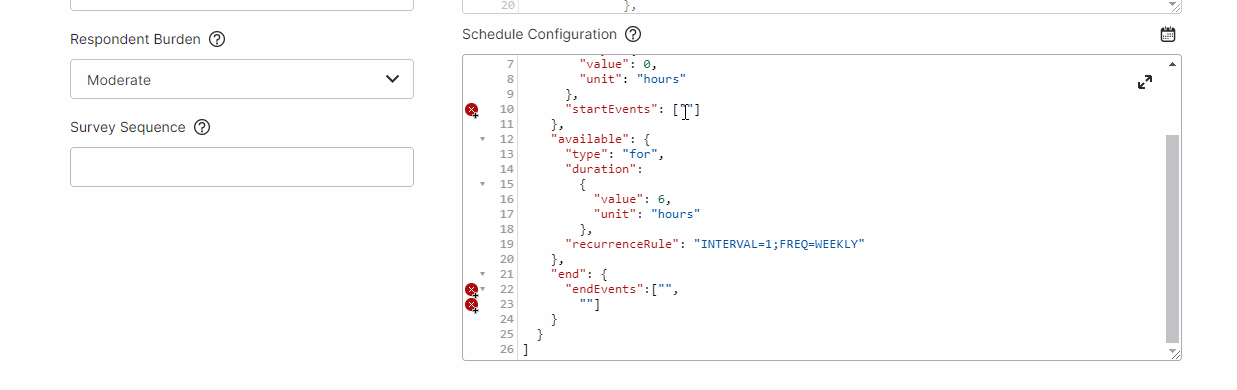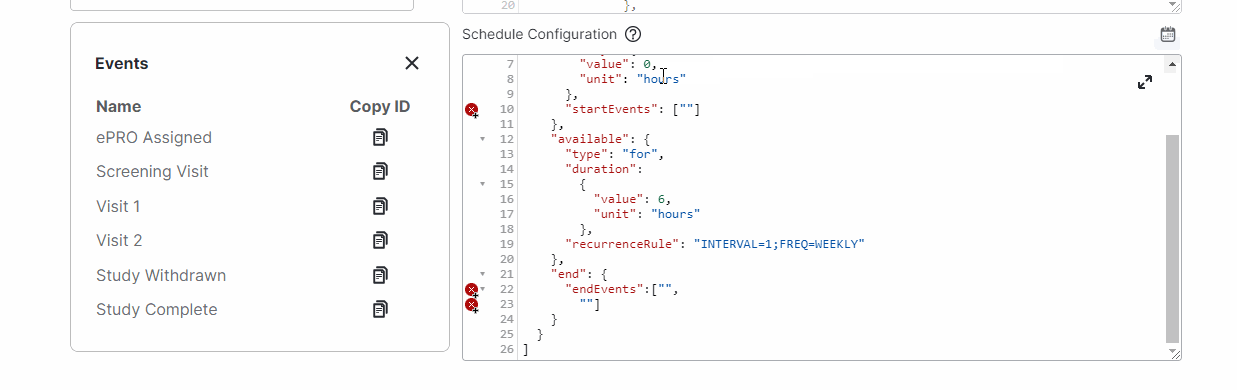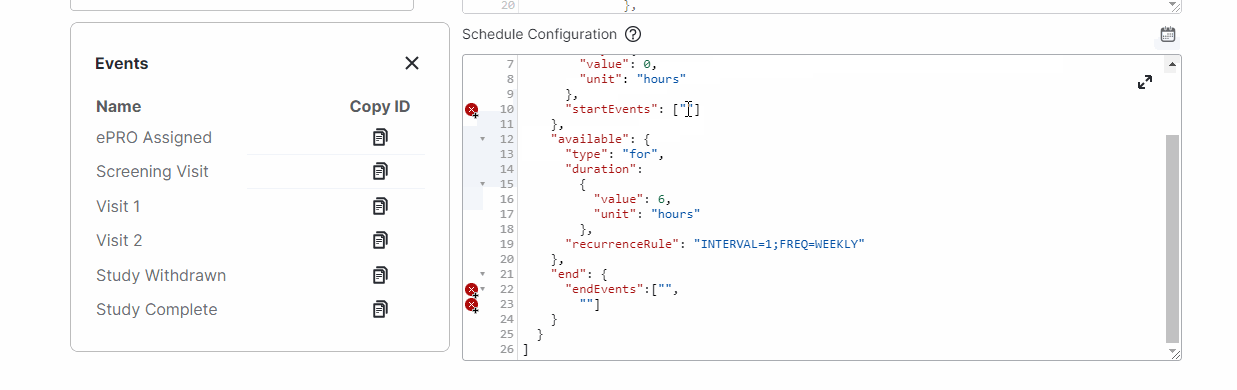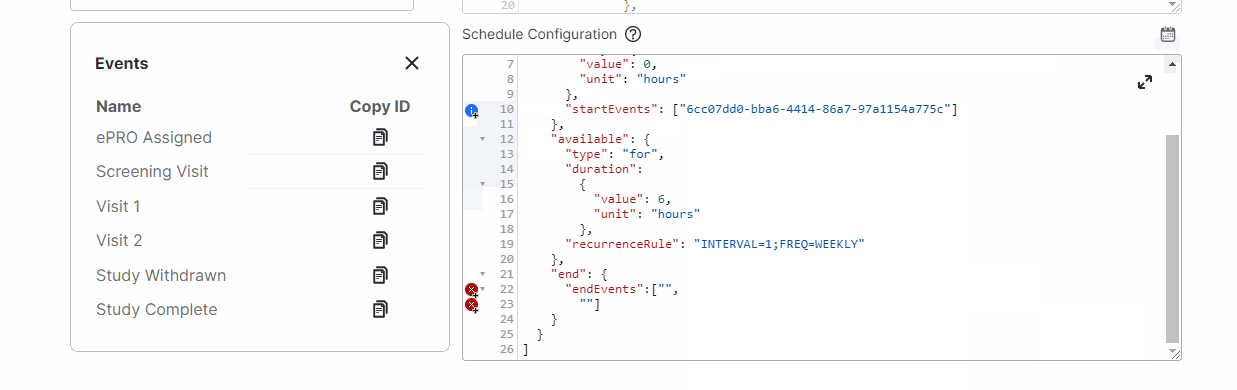
View Events While Editing Schedules
This feature allows study builders to view events and copy event IDs while editing a survey’s schedules by selecting a new calendar icon above the Schedule Configuration field.For more information, see Configuring Schedules and Notifications in Clinical Vault Help.
-
eClinRO - Collection Document Excludes eClinRO Surveys
This feature prevents collection documents from including information and screenshots about a study’s electronic clinician-reported outcome (eClinRO) surveys. Collection documents only contain information about participant-facing surveys and are intended to aid IRB/EC reviews.For more information, see Exporting a PDF Overview of a Collection in Clinical Vault Help.
-
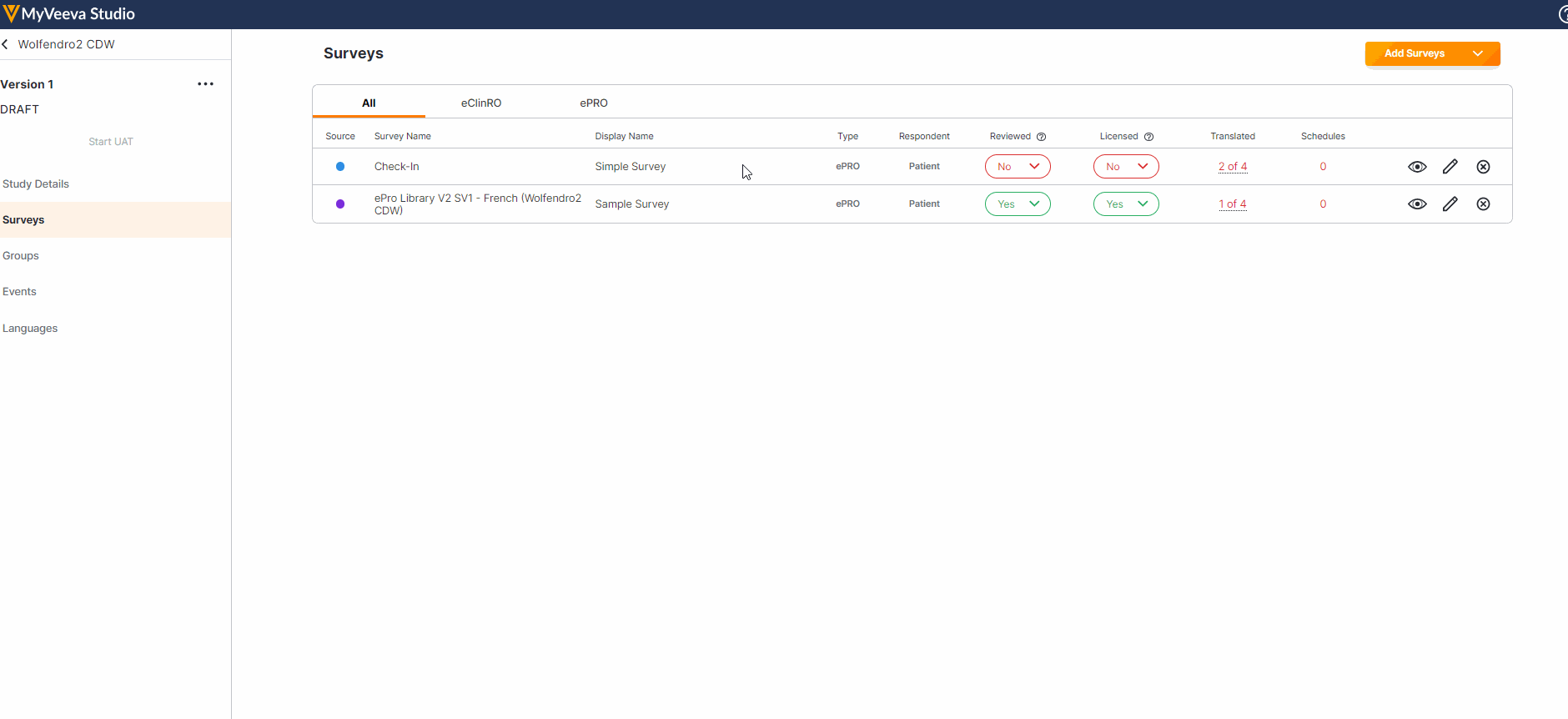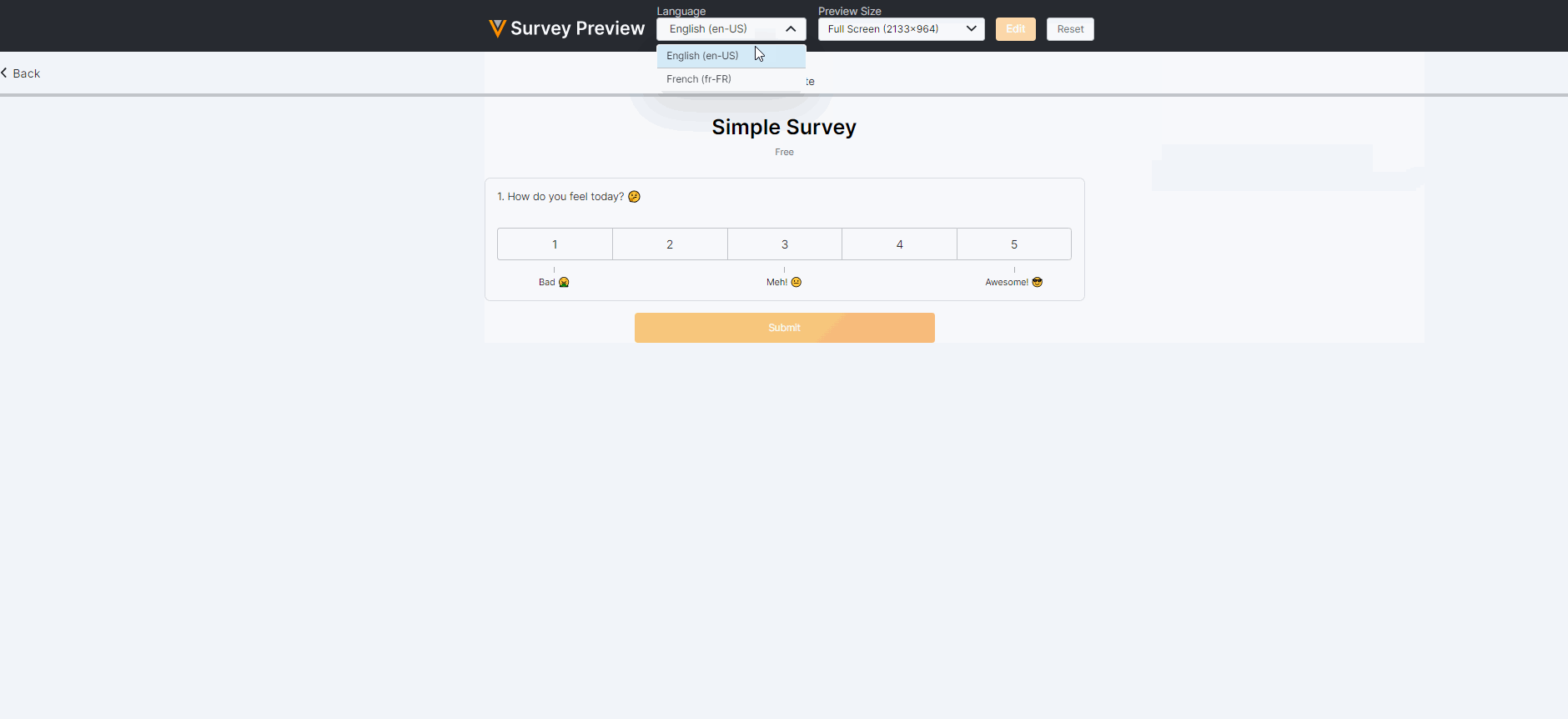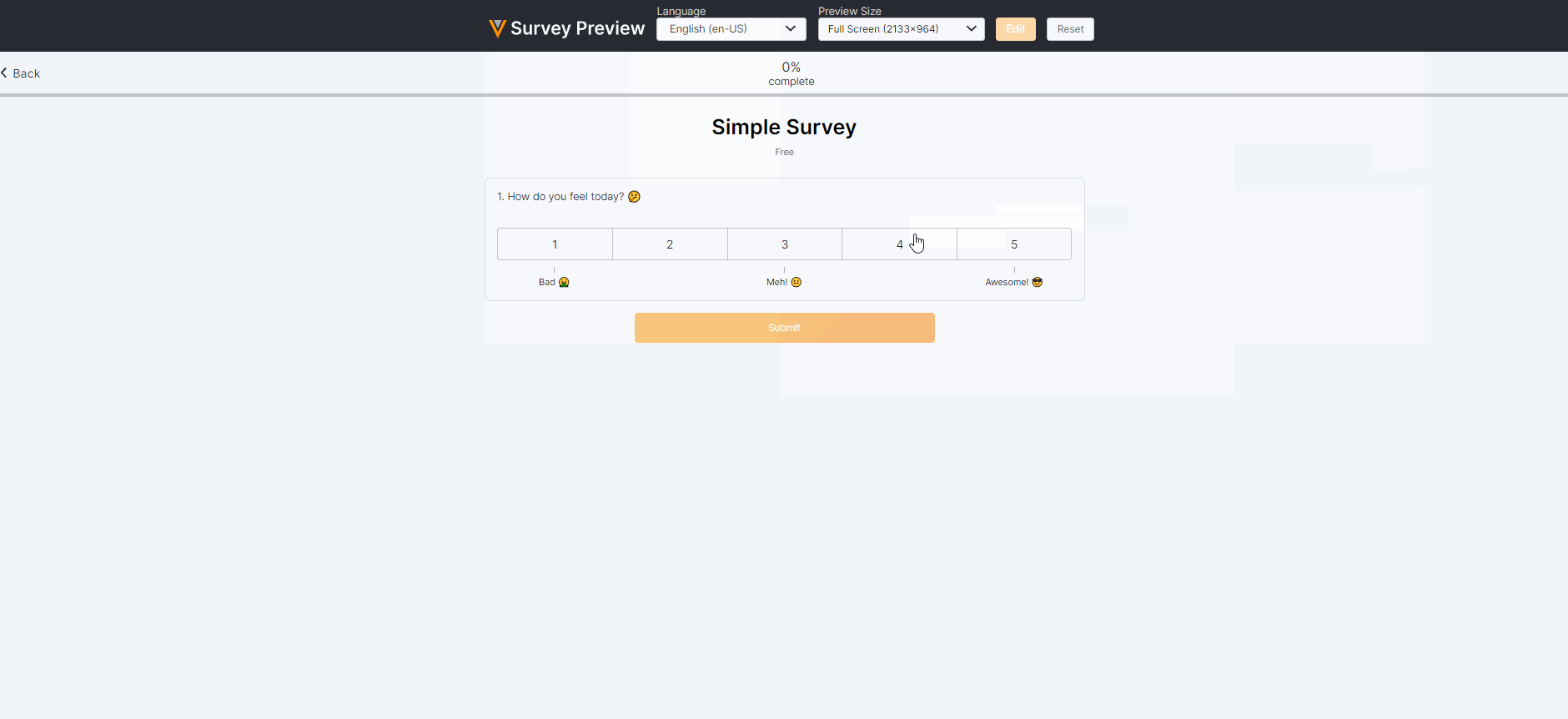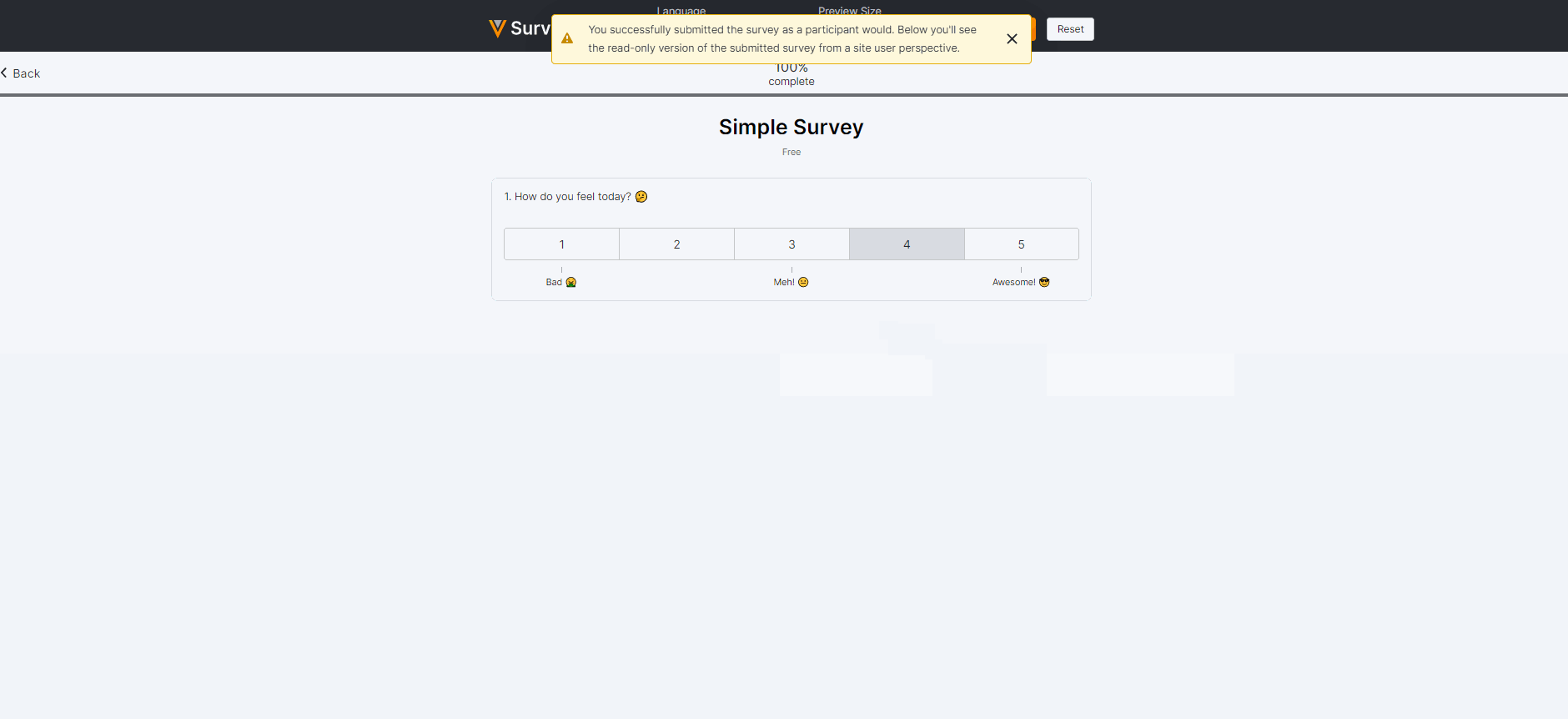
Survey Preview Enhancements
This feature enhances our survey previewer. Users can now edit or reset survey responses, view a read-only version of the survey after submission (similar to how a Site user would view the survey), and view/verify scoring calculations. The preview has also been optimized for mobile viewing.For more information, see Previewing a Survey in Clinical Vault Help.
Survey Enhancements
-
In-Person Survey Completion
This feature allows MyVeeva users to complete surveys in person at their study site. Study builders can now configure a new location parameter in a survey’s schedule JSON with “home” or “clinic” values to specify where MyVeeva users are expected to take the survey. Site users can start a participant’s in-person session from the participant event list in Study Connect. Participants can either complete their surveys on the site’s device or on a separate device. During an in-person survey session, MyVeeva users can complete any study surveys that are currently available to them. MyVeeva users are required to enter their date of birth and confirm their identity prior to beginning an in-person survey session.For more information, see Configuring Surveys in Clinical Vault Help.
-
Survey Scoring
This feature allows study builders to configure scoring for ePRO and eClinRO surveys. Study builders can assign numeric score values to single-choice, multiple-choice and optional answers. Numeric values from number entry, numeric rating scale, and visual analog scale question types can also be used in scoring. Study builders can configure one or more scores based on a respondent’s selected answers. If allowed, site users can see the calculated score of completed surveys. Study builders can test score calculations in the survey preview. Each calculated score is included in survey response data exports for sponsor and site users.For more information, see Configuring Survey Scores in Clinical Vault Help.
-
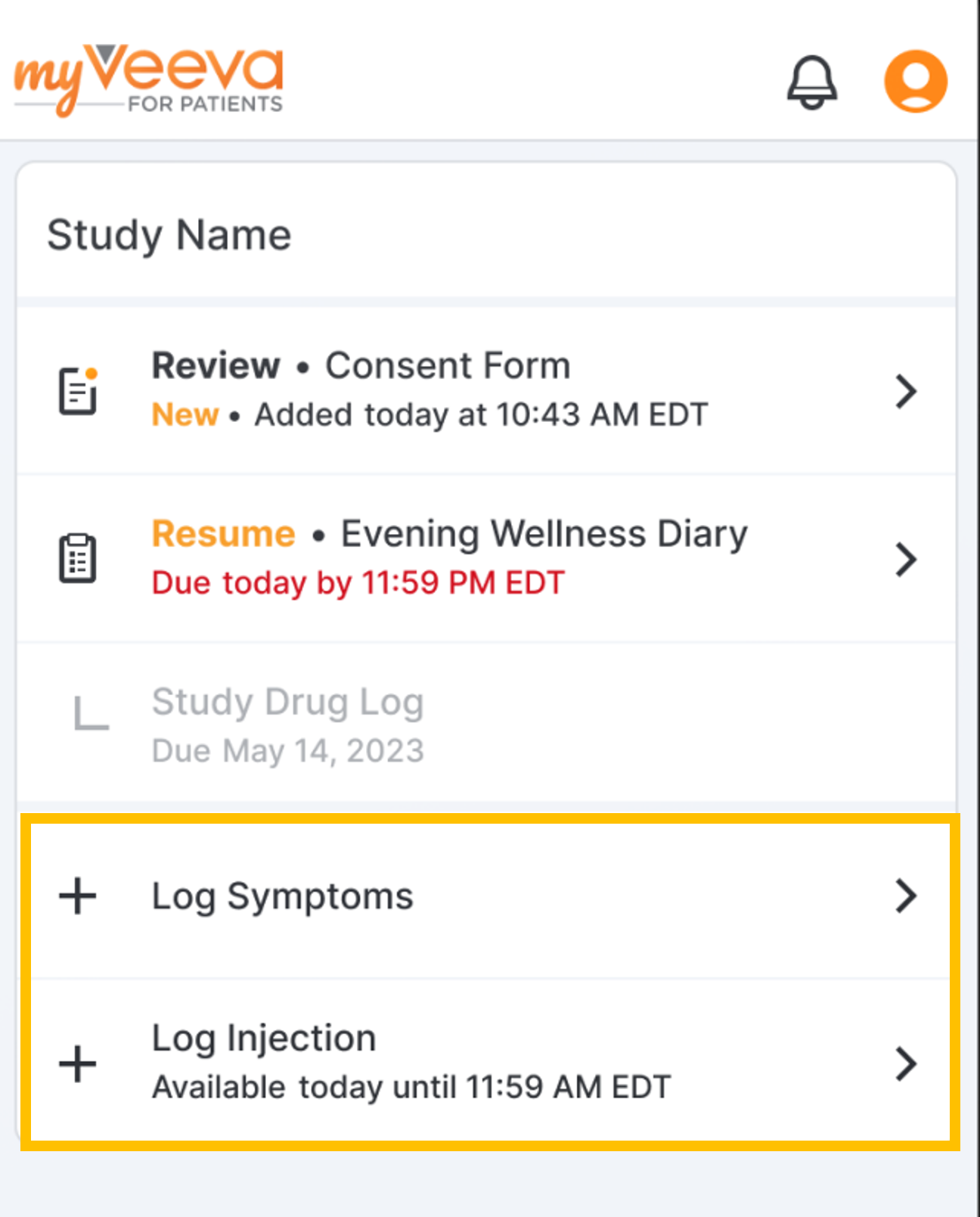
As-Needed Survey Enhancements
This feature enhances as-needed surveys for MyVeeva users. When study builders configure a survey with an as-needed schedule, they are now required to define an as-needed display name (such as “Log Drug Intake”) that instructs MyVeeva users when to complete the survey. The system also prevents users from being able to assign a survey sequence value to an as-needed survey. As-needed surveys are now clearly distinguished from other scheduled surveys on a MyVeeva user’s Tasks page. As-needed surveys in collections approved prior to 23R2 are not affected by these changes unless the survey is edited during a collection upversioning.For more information, see Configuring Surveys and Configuring Schedules in Clinical Vault Help.
-
Number Entry Component With Two Fields
This feature allows sponsor staff to configure and site users and participants to fill out two fields in the number entry question type on surveys. For example, to require and input hours and minutes. Currently, the number entry question type supports only one field. Similar to the single number entry field, site users and participants must enter numbers in each field that are multiples of the configured increments and within the configured minimum and maximum numbers.For more information, see Configuring Surveys in Clinical Vault Help.
-
Selectable Links in Surveys
This feature allows study builders to add selectable HTML web links to surveys for MyVeeva users to open. Study builders can add links to a survey’s license text and question headings.For more information, see Configuring Surveys in Clinical Vault Help.
Site Experience Enhancements
-
eClinRO - Site Actions
This feature allows site users to manage and submit electronic clinician-reported outcome (eClinRO) surveys in SiteVault. From a participant event in the participant record in SiteVault, site users can view a survey list related to that participant event and complete and submit the surveys. When a site user begins an eClinRO survey, it’s locked and made viewable in read-only format for other site users until the responding user submits, becomes inactive, or exits the survey. Site users can view completed eClinRO surveys, and audit trail data exports now include eClinRO-related events. Studies using only eClinRO surveys are not required to approve the collection document prior to enabling ePRO for the study. When upversioned collections include both ePRO and eClinRO surveys but only modify the eClinROs, the collection version for participants is automatically updated. -
eClinRO and ePRO - Participant Event Enhancements
This feature provides site users with an enhanced participant event list in Study Connect. They can drill into a participant event to view all related ePRO and eClinRO surveys. Each survey is identifiable by its status, and the user can begin new or view completed surveys. Additionally, users can toggle the list to view only the available surveys. When entering eClinRO surveys or viewing completed eClinRO or ePRO surveys, the site user can switch between the languages that the survey supports.

- Survey Compliance Warnings for Sites
This feature allows site users to view survey compliance warnings in Study Connect. When participants have important surveys coming due or have missed surveys in the last seven days, a new widget on the Surveys Overview page displays detailed information about compliance for those participants. From the widget, site users can access participant contact information and add notes. Compliance warnings for surveys that are coming due soon are derived from the survey’s “Survey Due” site notification configuration, which is defined by a study builder.
Additional Enhancements
ePRO Survey Report for Monitors in Clinical Operations
This feature provides sponsor users with a report that can be used to monitor ePRO-connected studies in Clinical Operations. From the Study Site or Monitoring Event records, sponsor users can generate and export a ZIP file containing a site’s survey responses, survey compliance, audit trail, compliance summary, and SiteVault user access data.
A sponsor must have Vault CTMS to execute the action on a Monitoring Event.
For more information, see ePRO Survey Report for Monitors in the Clinical Vault Release Notes.