SiteVault Release Webinar Recording
SiteVault Release Highlights
SiteVault eReg
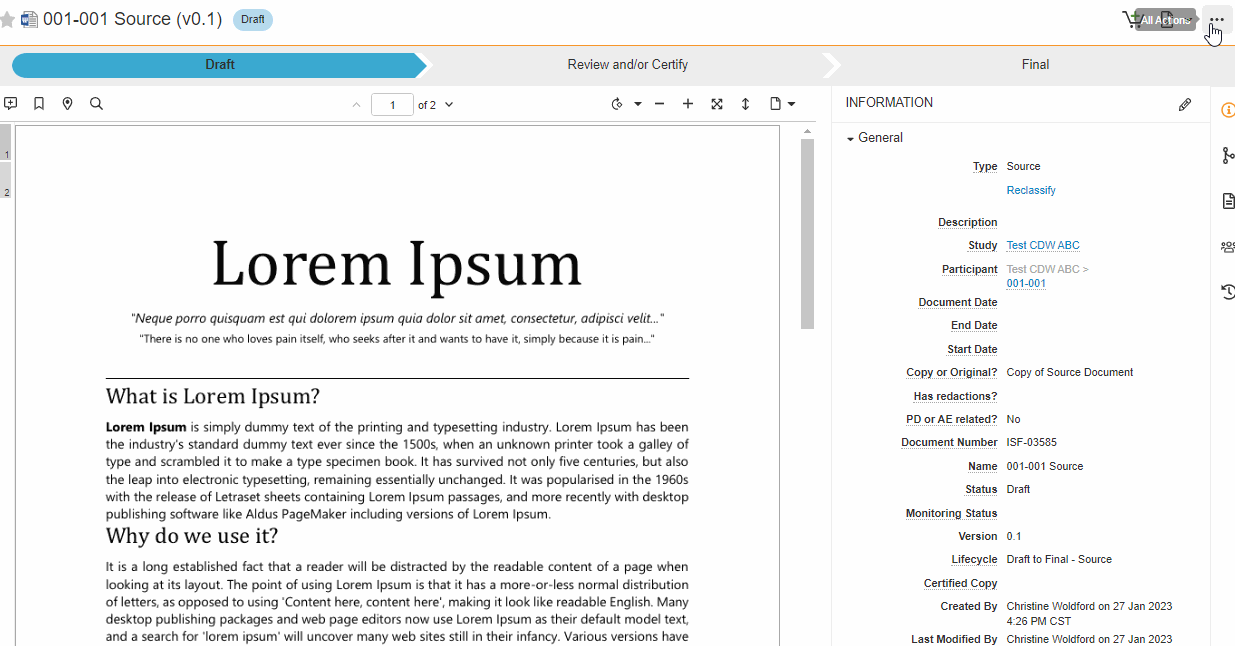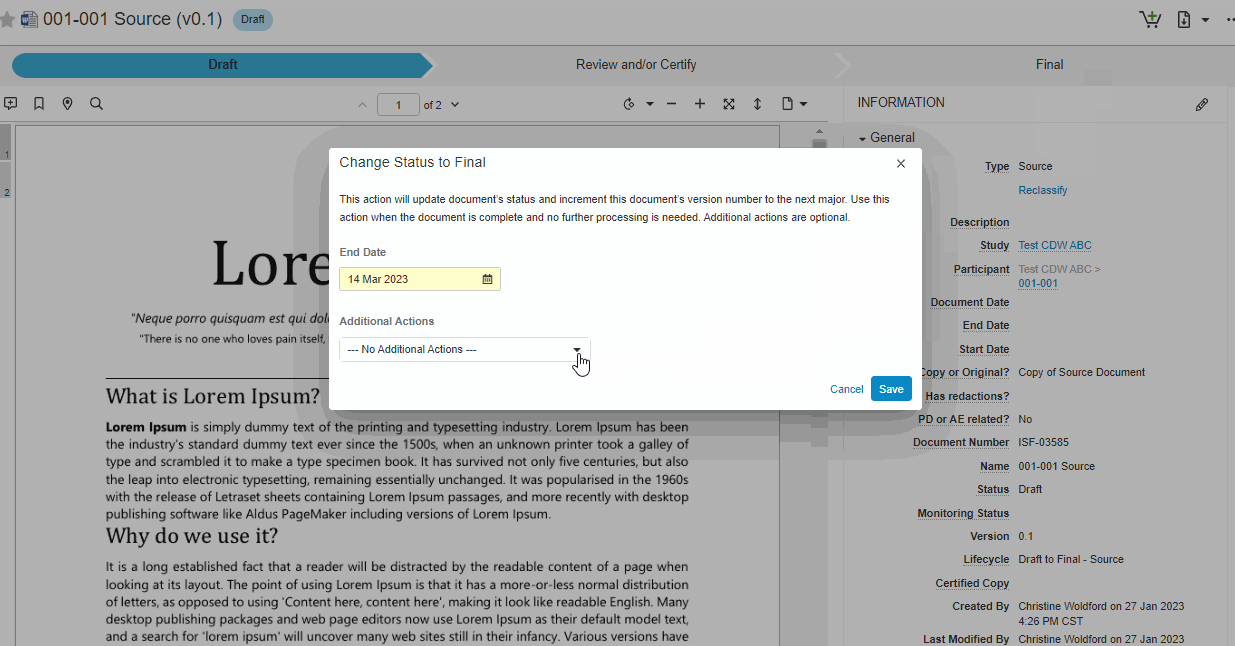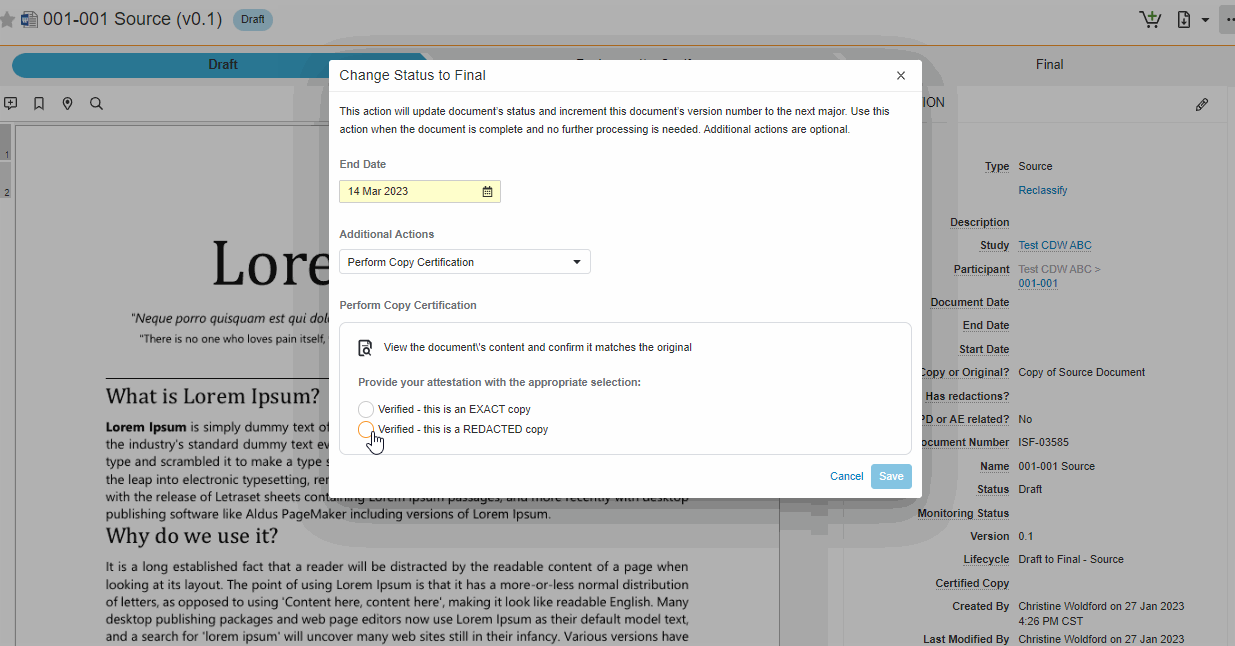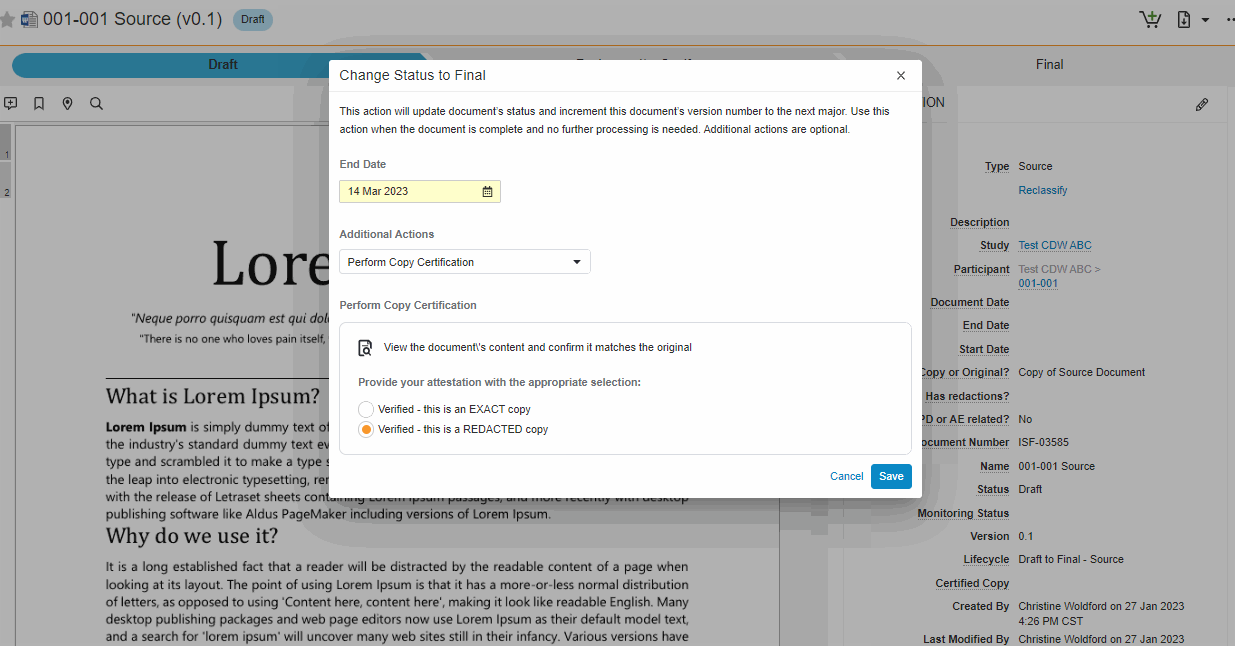
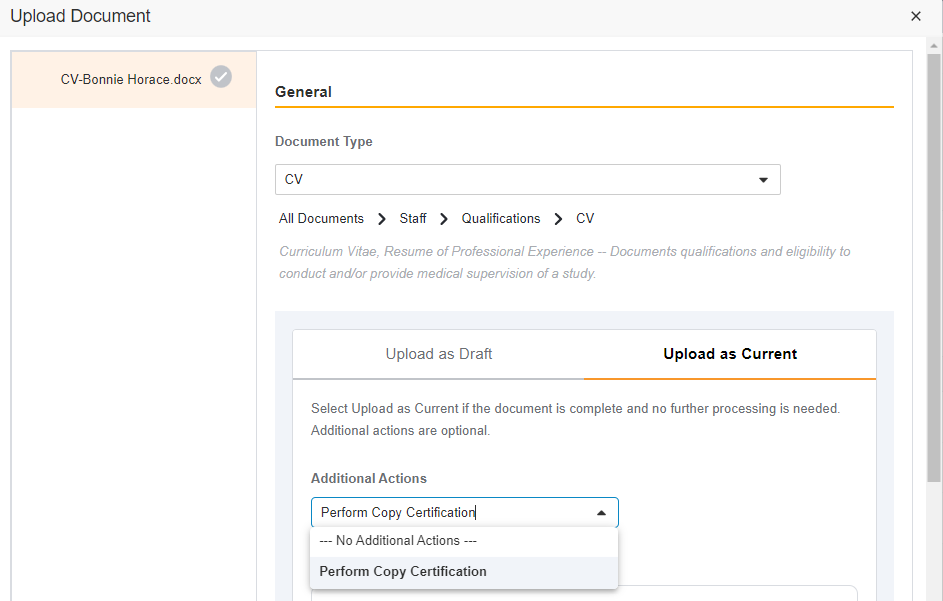
Single Action for Document Status Change and Certify as Copy
This feature brings consistency to finalizing documents across all document types in SiteVault eReg. We’ve replaced the Change State to (Final State), Finalize Source, and Certify as Copy workflows with a single all-in-one action. The new all-in-one document actions give users the ability to change the status of a document and perform the copy certification process in one easy flow.
Note This new action can be accessed under the Document or All Actions menus.
Study eBinder Enhancements
This feature increases eBinder functionality and ease of use:
- Drag and Drop Documents into Study eBinder Document Grid
With this feature, you can upload a document to the Study eBinder by dragging a file into the document grid (table).
- Folders with Document Indicators
With this feature, eBinder will include a document indicator and a document count to help you easily identify which folders contain documents.

- eBinder Upload to Current/Approved/Final State
This feature gives you the ability to upload documents directly into their steady state.
Document Workflow Enhancements
This feature offers new document workflows and a streamlined process for existing document workflows.
- Generic Review and eSignature workflows
This feature introduces two standard workflows:
- Review: The Review workflow allows you to request others to review a document and provide feedback by using the annotation tool. The Review workflow is available on the following lifecycles: Draft to Approved for Use, Draft to Current, and Draft to Final.
- eSignature Approval Workflow: eSignature Approval allows you to collect eSignatures on documents requiring one or more signatures for approval. The eSignature Approval workflow is available on the following lifecycles: Draft to Current and Draft to Final.
- Review: The Review workflow allows you to request others to review a document and provide feedback by using the annotation tool. The Review workflow is available on the following lifecycles: Draft to Approved for Use, Draft to Current, and Draft to Final.
- Draft to Approved for Use Lifecycle Usability Enhancements
This feature provides an updated look and feel for documents using the Draft to Approved for Use lifecycle.
Monitor & External User Access Management
These enhancements improve the user administration experience while using the Access and Permissions section of the Monitors and External Users tab:
-
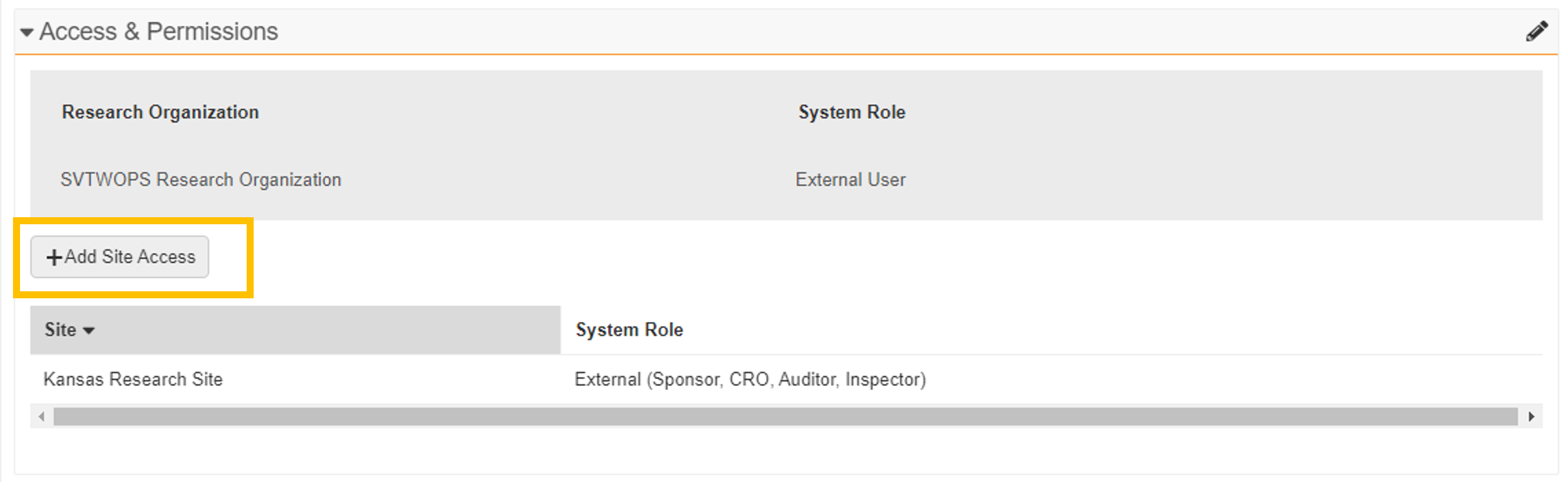
Site List
For multi-site research organizations, only sites to which the monitor or external user currently has access are displayed in the section. -
+Add Site Access
For multi-site research organizations, the +Add Site Access button can be used to add the monitor or external user to an additional site.
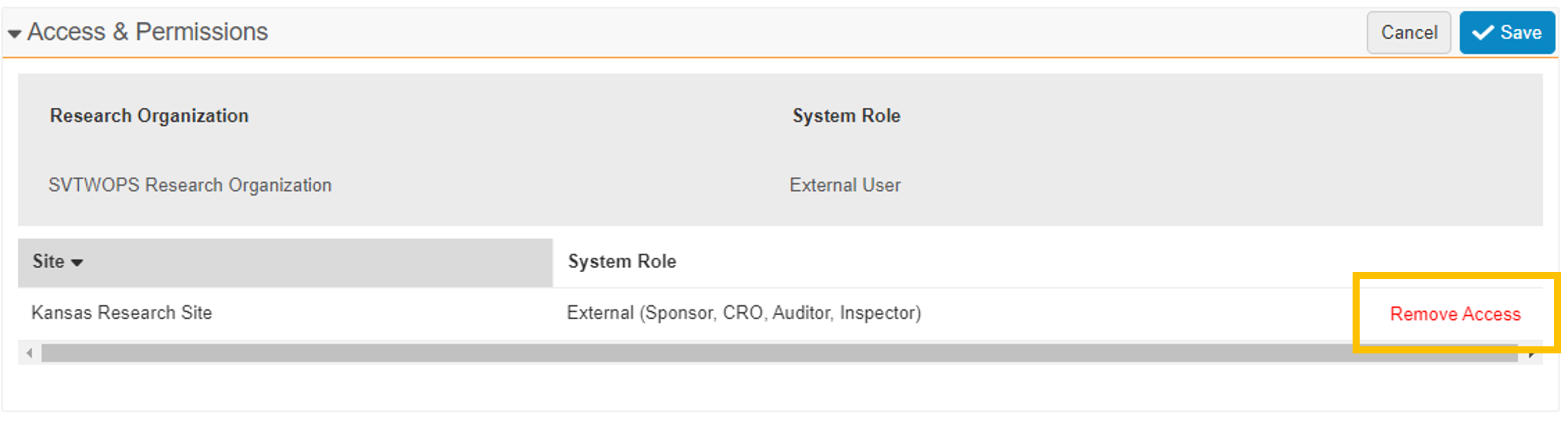
- Remove Access
The Edit User Permission button has been replaced with a pencil icon. When selected, the pencil icon puts the section into Edit mode. While in Edit mode, a Remove Access action is available for any of the listed sites. This action also changes the user’s study assignments to Inactive. When all site access has been removed, the user record automatically changes to Inactive.
- Automated Study Assignment Status
The Monitor/External User study assignment status is automatically updated to Active when you add a Monitor/External User to a new study (either on the Studies tab or the Monitors and External Users tab), unless you provide a future Scheduled Start Date. Previously, such new study assignments began in the Proposed status. The Monitor/External User study assignment status is automatically updated to Inactive when site access is removed (when a user selects Remove Access).
Attachments Available on all Document Types
Attachments provide a simple way to upload and associate related files with existing documents (for example, certificates of translation for participant-facing materials). With this change, the Document Types spreadsheet will no longer include a column for identifying document types that allow attachments.
Digital Delegation: Flexible Workflows and Start/End Dates
This feature includes multiple changes that add flexibility to the Digital Delegation set-up process.
- Start and End Date Fields
- Various Start and End date fields used with Digital Delegation have been re-labeled for clarity.
- Staff member Start and End Date fields used with Digital Delegation can be edited manually.
- PI Approval Flexibility
PI Approval can be initiated at any point, if at least one staff member has completed acceptance.
For more information, see Sending Delegations for Acceptance and Approval.
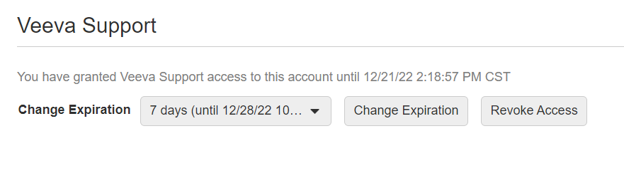
Grant Veeva Support Access to SiteVault
This feature enables you to grant Veeva Support access to your SiteVault account. When you need assistance from Veeva Support, it can be useful for them to see your SiteVault from your perspective; this Profile tool is used to set a timeframe for Support to access your SiteVault.
For more information, see Managing Your Profile.
Language Label Change: Spanish to Spanish (Spain)
This feature does not change any technical functionality, but adds clarity to language labeling. The Spanish language options for patients and patient-related materials are Spanish (Spain) and Spanish (Mexico). All patients and related patient-related material that are currently assigned the Spanish language will be assigned Spanish (Spain).
- Previous Spanish Labels
- Spanish
- Spanish (Mexico)
- New Spanish Labels
- Spanish (Spain)
- Spanish (Mexico)
MyVeeva Patient Password Reset
With this feature, Site Admins and Site Staff working in eReg can initiate a password reset on behalf of a MyVeeva user.
Participant Enhancements (eConsent and ePRO)
These enhancements improve the user experience when managing Participants in eReg:
-
Participant Visit Scheduling
Note: This feature requires the patient to have a MyVeeva account. This feature enables you to invite participants to upcoming visits using calendar applications such as Outlook and Google Calendar directly from SiteVault. When you include the MyVeeva calendar alias in your invitation, the event will be available to patients in MyVeeva for Patients. -
Prevent Further Changes to a Participant’s Patient ID
This feature prevents you from changing a study participant’s Patient ID after the participant is invited to use MyVeeva for Patients. -
Participant ID Harmonization
On a Veeva EDC-enabled Connected Study, when you create a new participant record or edit an existing Participant ID, an automated check determines if a Participant ID is present in Veeva’s EDC. Study Connect users can view the participant’s EDC status in the EDC Status field, on the Participant record, along with a link to the participant’s casebook in EDC.
eConsent Enhancements
These enhancements improve the user experience within the SiteVault eConsent functionality:
- Automatically Close eConsent Editor Session and Refresh SiteVault Session Upon Checking In eConsent Form to Vault
This feature makes the following enhancements to the eConsent form check in process (from eConsent editor to Vault):- When you check in an eConsent form, the editor window automatically closes.
- When you check in an eConsent form, the page that launched the editor (either the eReg Document Viewer or the Study Connect Blank Forms) automatically refreshes with the eConsent form’s new content.
- Approve and Send Site-Specific eConsent Form Template to Sponsor/CRO in SiteVault eReg
This feature allows you to approve and send a connected eConsent form to the study’s sponsor/CRO in SiteVault eReg after making site-specific edits in the eConsent editor.
- Create New Draft of Connected eConsent Template in Study Connect
This feature allows you to create a new draft of a blank, connected eConsent form template by:- Creating a copy of the sponsor template in Study Connect
- Creating a copy of the most current version in Study Connect
- Uploading a file from your computer in Study Connect
SiteVault Study Connect
Grid Column Ordering and Name Column Locking
The following enhancements improve the user experience within the Study Connect main grids (tables):
-
Fixed First Columns
When you scroll to the right, the first column in the grid remains fixed in place. -
Flexible Column Order
You can reorder the grid columns on certain pages by dragging the column header.
Notification Bell for Document Exchange, Safety Distribution, and eConsent
With this feature, you can view notifications from any study-specific Study Connect tab. When the Notifications Bell is selected, a panel of notifications is displayed on the right, sorted to notifications related to the feature currently selected in the Study Connect main menu. Filters can then be removed or added as necessary.
Document Exchange Enhancements
The following enhancements improve the user experience within the Document Exchange functionality:
- Sponsor/CRO Document Name
On a received document, you can view the name of the document as it appeared in the sponsor/CRO Vault. This field can be viewed in the All Documents and Received Documents tabs of Document Exchange.

-
Sponsor/CRO Document Number
On a received document, you can view the number of the document as it appeared in the sponsor/CRO Vault. This field can be viewed in the All Documents and Received Documents tabs of Document Exchange. -
Document Version on Send
When sending an eReg document, the version number of the document is displayed in the document name. -
Link to eReg Document
With this feature, you can now quickly access a Study Connect’s associated eReg document copy. An eReg document icon is available in the Document Actions, All Documents, Sent Documents, and Received Documents tabs of Document Exchange.
ePRO Collection Document Requiring Action Displays Visual Indicators in Study Connect
With this feature, you are now notified when an ePRO collection document requires attention. A blue dot indicator on the Studies tab and an orange Action Needed icon on the ePRO tab are displayed when an ePRO collection document requires action from a site user.
Approve and Send Site-Specific eConsent Form Template to Sponsor/CRO in Study Connect
This feature allows an eConsent form template that has been edited in the eConsent editor to be approved and sent to the connected study’s sponsor/CRO in Study Connect.
Participant Profile Enhancements
The following enhancements improve the user experience within the Participant tab:
- Participant ID Harmonization
On a Veeva EDC-enabled Connected Study, when you create a new participant record or edit an existing Participant ID, SiteVault checks if a Participant ID is present in Veeva’s EDC. The participant’s Veeva EDC Casebook status is displayed in the Veeva EDC Casebook field.- Veeva EDC Casebook Field: This new field provides the status of the automated check. The following statuses are available:
- Searching: The system is still actively conducting the automated check.
- Open Casebook: A casebook matching the Participant ID was found and those with the required permissions can click the status to link directly to the casebook in the sponsor’s Veeva EDC system.
- Not Found: The Participant ID was not located in Veeva’s EDC.
- Veeva EDC Casebook Field: This new field provides the status of the automated check. The following statuses are available:
-
Participant Visit Scheduling
Note: This feature requires the patient to have a MyVeeva account. This feature enables you to invite participants to upcoming visits using calendar applications such as Outlook and Google Calendar directly from SiteVault. When you include the MyVeeva calendar alias in your invitation, the event will be available to patients in MyVeeva for Patients. -
Countersign eConsent Form From Participant Profile in Study Connect
This feature allows you to countersign an eConsent form from the consenting participant’s profile page. -
Cancel Signed eConsent Forms From Participant Profile in Study Connect
This feature allows you to cancel a signed eConsent form from the consenting participant’s profile page. -
Description Column for Signed Forms on Participant Profile
This feature adds a Description column to the Signed Forms section on the participant’s profile page. -
Participant Medical Record Number Field
This feature adds a Medical Record Number field to the participant’s profile page. - Pagination Added for Ease of Use
- Form Responses
- Signed Forms
- Participant Events