SiteVault
This release introduces a whole new way to manage your eRegulatory documents and exchange study information with sponsors in SiteVault.
For SiteVault customers currently using SiteVault as an eRegulatory solution, SiteVault is now known as SiteVault eRegulatory and includes a refreshed Study eBinder where study staff and monitors can manage study-related documents all in one place.
For SiteVault customers who exchange documents with sponsors and CROs on connected studies, SiteVault Study Connect offers an enhanced place for sites to securely access and exchange trial information.
SiteVault Study Connect
SiteVault Study Connect - Available December 3, 2022

Study Connect is a new application in SiteVault where site users can complete all of the necessary sponsor-driven activities to effectively conduct a connected study. The Study Connect application is available to sites that are invited to a connected study by a sponsor.
Study Connect includes a tailored user experience for each of the following workflows:
- Document Exchange: Receive and exchange study documents to reduce manual steps and redundant requests.
- Safety Distribution: Receive and acknowledge safety reports in a timely manner.
- eConsent: Provide participants with convenient access to consent forms and key study information.
- ePRO: Capture and manage study participant electronic patient-reported outcomes (ePRO).
With the implementation of Study Connect, you’ll find the following changes:
- Document exchange and safety distribution workflows are now located in Study Connect.
- eConsent and ePRO workflows are now located in Study Connect. Note: Site users on an eConsent- or ePRO-enabled connected study before this release can continue using SiteVault eRegulatory, but shifting to Study Connect will provide a more streamlined experience.
- For SiteVault customers using both applications, you can use the Switch to Study Connect and Switch to eReg buttons in the upper-right corner to navigate between the applications.
- The Regulatory Document Request page is retired as the functionality is now part of Study Connect.
- You now must send person and organization profile documents to a sponsor or CRO using the Send action on the document in Study Connect. With this release, these documents are no longer transferred automatically when a study team assignment or study partner organization are made active on a study.
New Exchangeable Document Types on Connected Studies
This feature adds Participant Adverse Event Log and Protocol Deviations to the list of available document types that can be exchanged with a sponsor or CRO through Study Connect.
This feature also adds support to receive Adverse Event Form templates from sponsors and CROs.
SiteVault eRegulatory
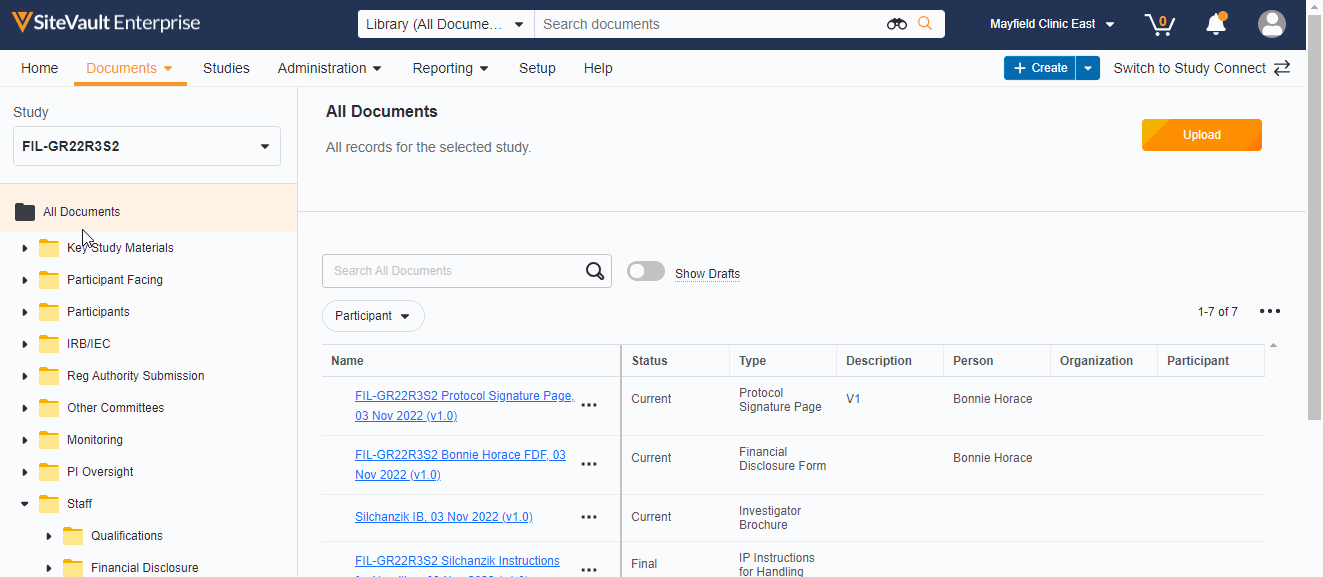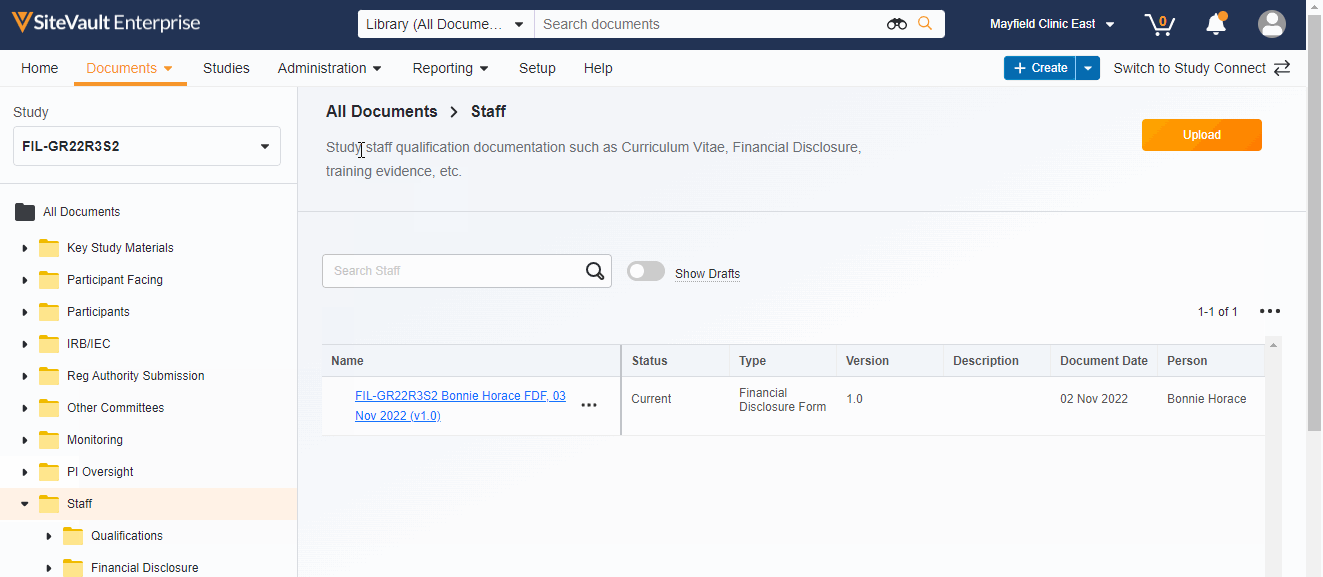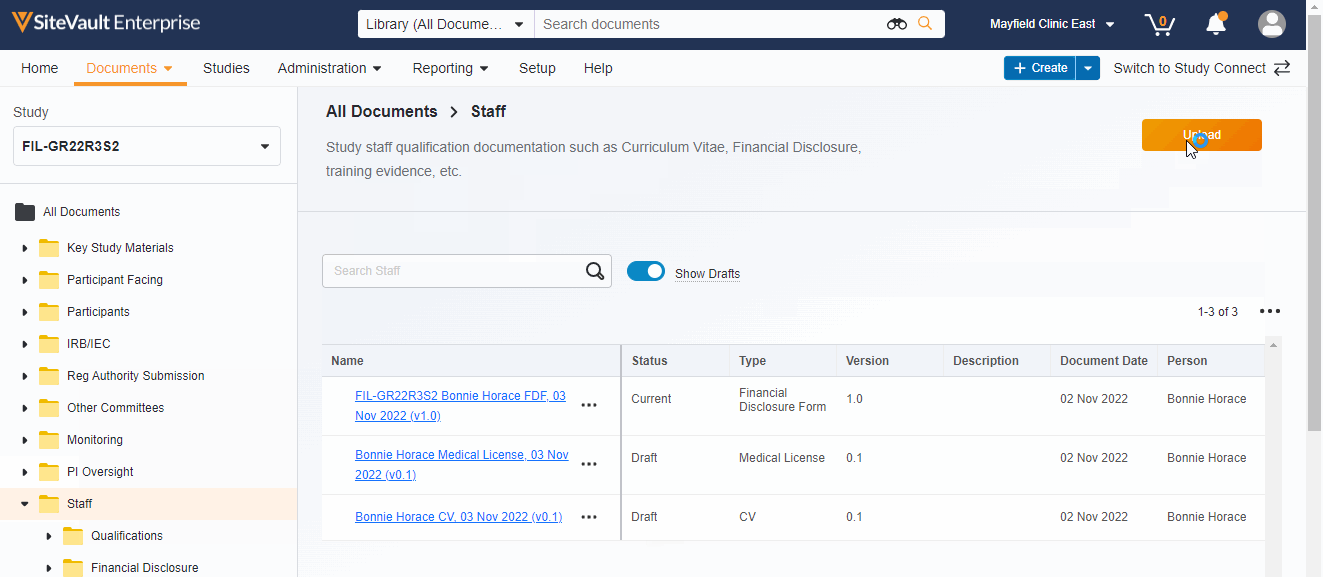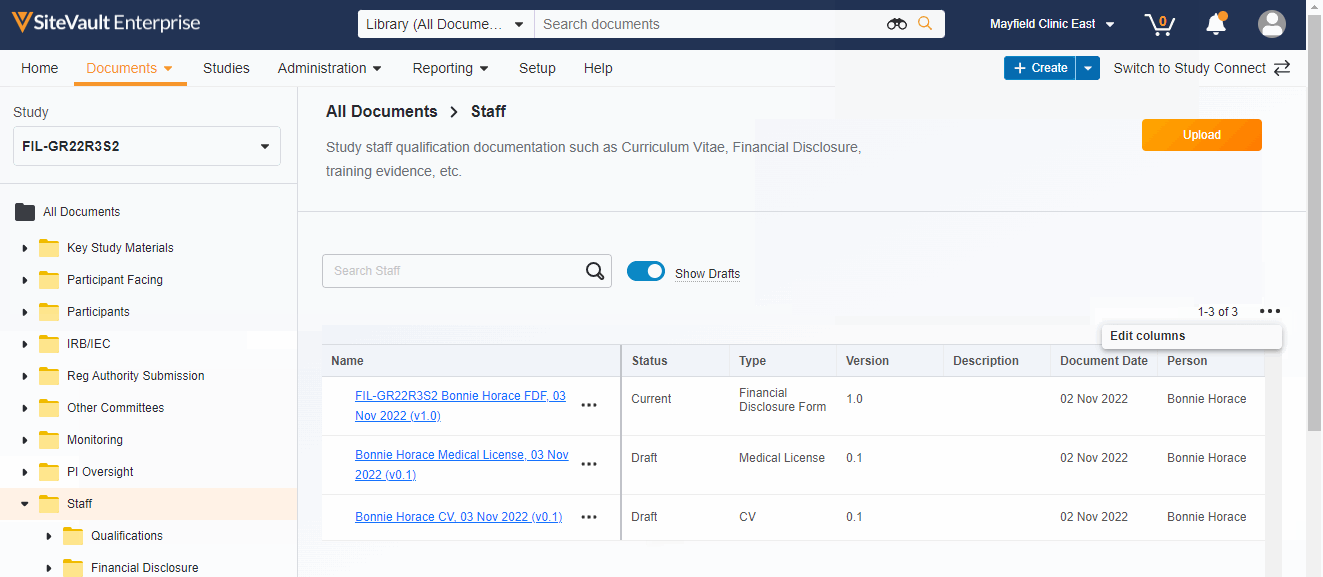
Study eBinder Redesign
This feature enhances the Study eBinder with a more modern look and feel. It also introduces the following usability enhancements:
- Document type-level folders
- Descriptions of document folders and document types
- The system displays help information when you position your pointer over certain elements in the Study eBinder
- Ability to display or hide draft documents
- Documents can be uploaded directly to the Study eBinder using a new Upload button or by dragging a document into a folder
Monitor & External User Access
With this feature, adding a monitor or external user to a specific site or study has been streamlined into a single process. You can now create accounts for your monitors and give them access to your studies all in the new Monitors & External Users tab.
As part of this change, we’ve updated the name of the Profiles tab to Administration and the name of the Users tab to Staff and removed references to external users from the Staff and Setup Assistant pages. We’ve also made the following miscellaneous updates:
- Study Assignment Fields Renamed
- Access Start Date is updated to Scheduled Access
- Start Access End Date is updated to Scheduled Access End
- Lifecycle State is updated to Assignment Status
- Status is updated to Record Status
- All active user profile records are automatically updated to reflect the following:
- External User Profiles
- User Profiles is updated to Monitor/External User
- Updated page layouts and default list columns
- The Organization field is displayed on monitor or external user profiles
- Internal User Profiles
- User Profiles is updated to Staff
- Updated page layouts and default list columns
- External User Profiles
Digital Delegation: Support for Site Staff without User Accounts
This feature relaxes data validation behavior on Digital Delegation studies by granting more flexibility in managing users without SiteVault accounts, and by allowing users to initiate PI Approval when one or more staff member acceptance is outstanding.
For more information, see Digital Delegation.
Update eConsent Workflows to Support Paper-Signed Forms and Additional Review
Note This feature is available in both SiteVault eRegulatory and SiteVault Study Connect for Veeva eConsent or Veeva ePRO customers.
This feature makes the following enhancements to the eConsent countersignature workflow:
- For paper-signed eConsent forms uploaded to SiteVault, you can now certify as copy and countersign the form in SiteVault.
- When countersigning an eConsent form, you can now assign an additional reviewer to review the form.
Reason for Change Required for Specific Participant-Related Updates
Note This feature is available in both SiteVault eRegulatory and SiteVault Study Connect for Veeva eConsent or Veeva ePRO customers.
With this feature, the system requires you to enter a reason for change when you update a participant ID or the actual date and time on participant events. This information is documented in the system’s audit trail.
For additional information on this exciting release, please visit the SiteVault 22R3 Release Hub.