Digital Delegation: Usability Optimizations
This feature introduces the following enhancements to Digital Delegation in SiteVault:
- Easy Enablement and Disablement: When you turn Digital Delegation on or off, you now see a confirmation message allowing you to proceed or cancel the action. When enabled or disabled, non-Principal Investigator study assignments reset to the Proposed state and any Digital Delegation workflows are canceled. This enablement and disablement reset action offers a refreshed Digital Delegation environment at any point during the study, eliminating the need to reset or cancel activity manually.
- Data Validation: Enhanced logic is applied to prevent common mistakes and avoid unnecessary workflows and tasks. For example, when you take an action that can’t be completed because of a missing setting, an error message is displayed that notifies you of the setting that requires attention.
- Bulk Edit Staff Delegations: You can now add and remove multiple delegations for a staff member at once, simultaneously add or remove multiple staff delegations, and edit delegations without leaving the study person details page.
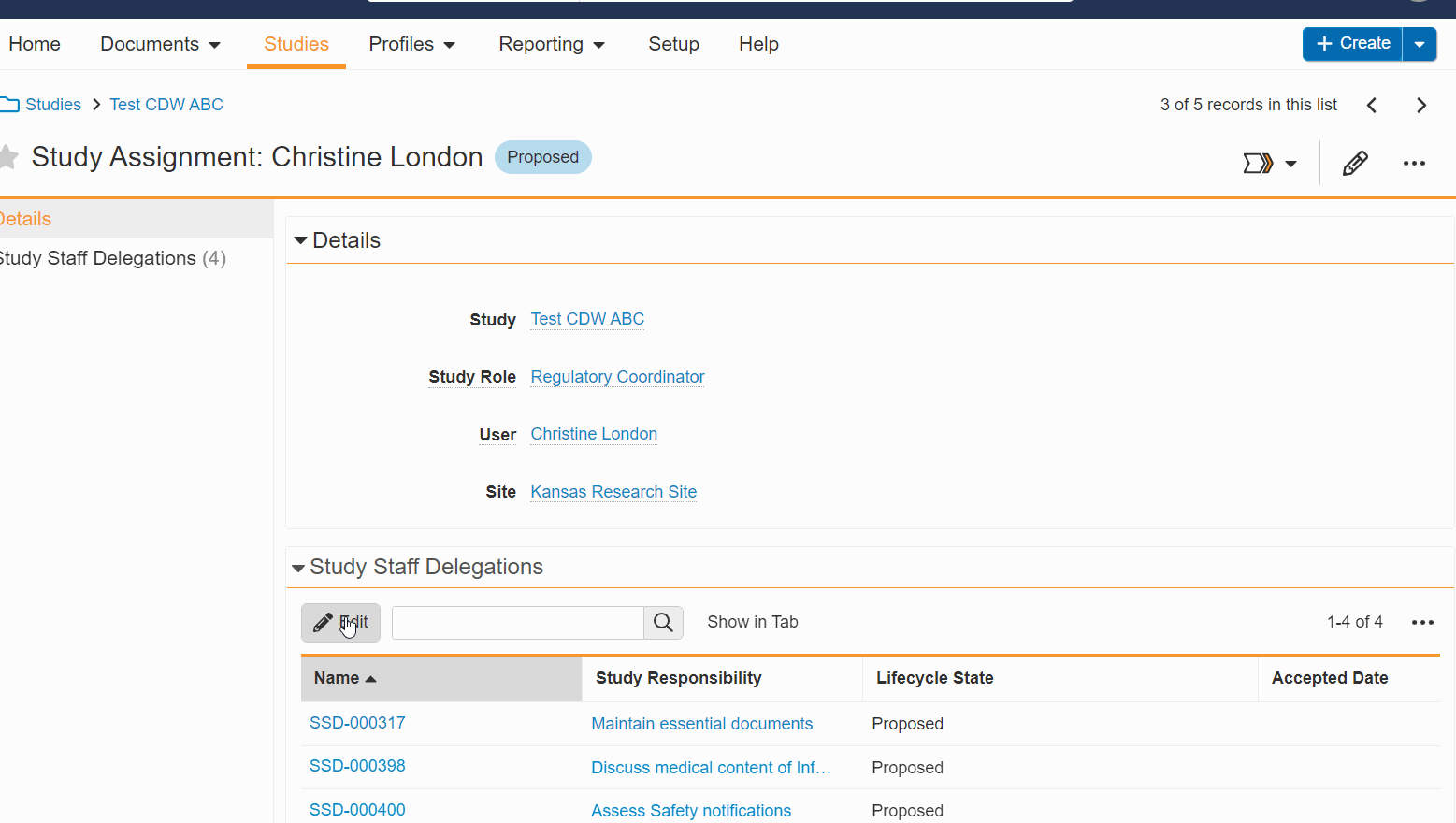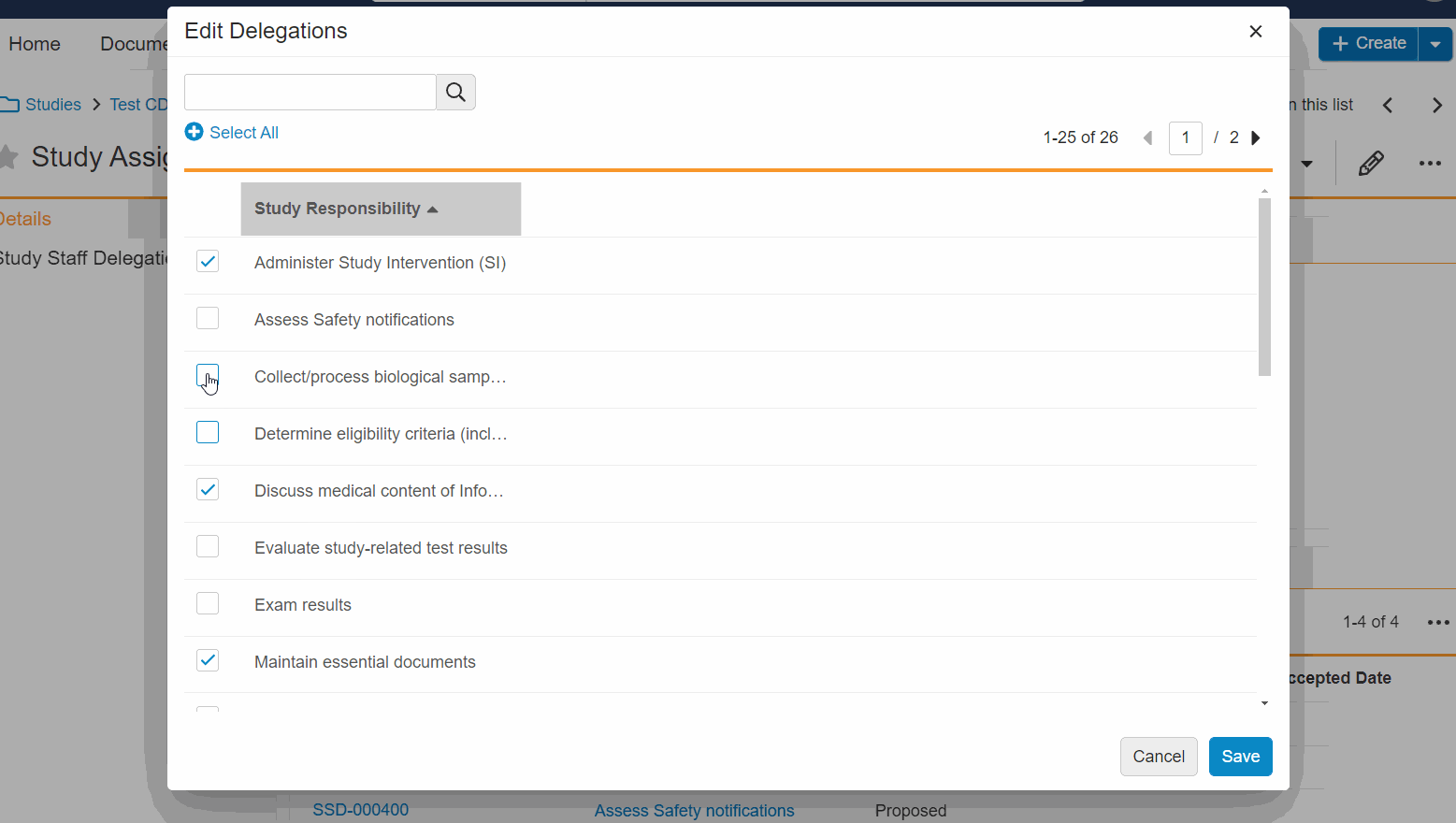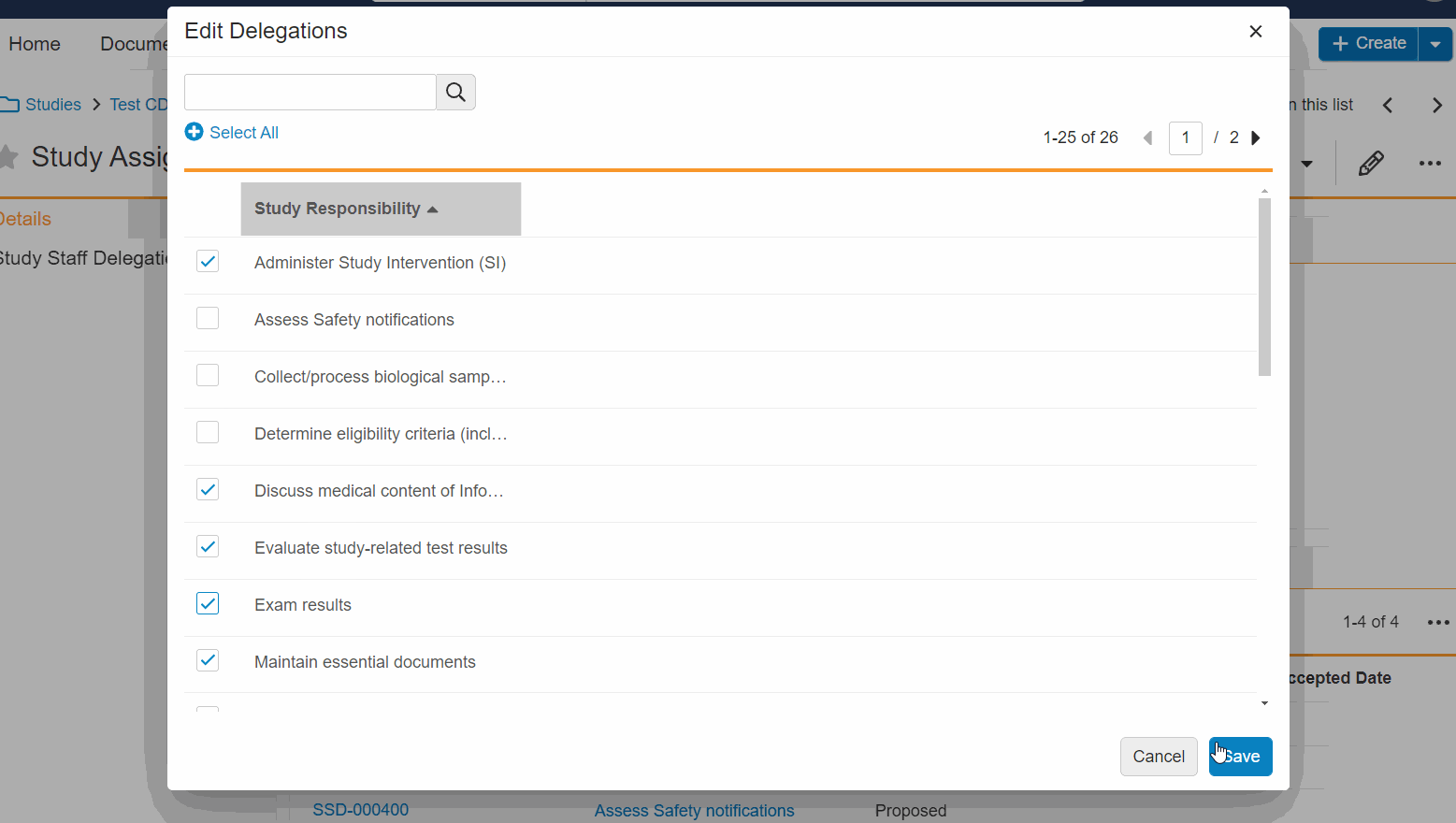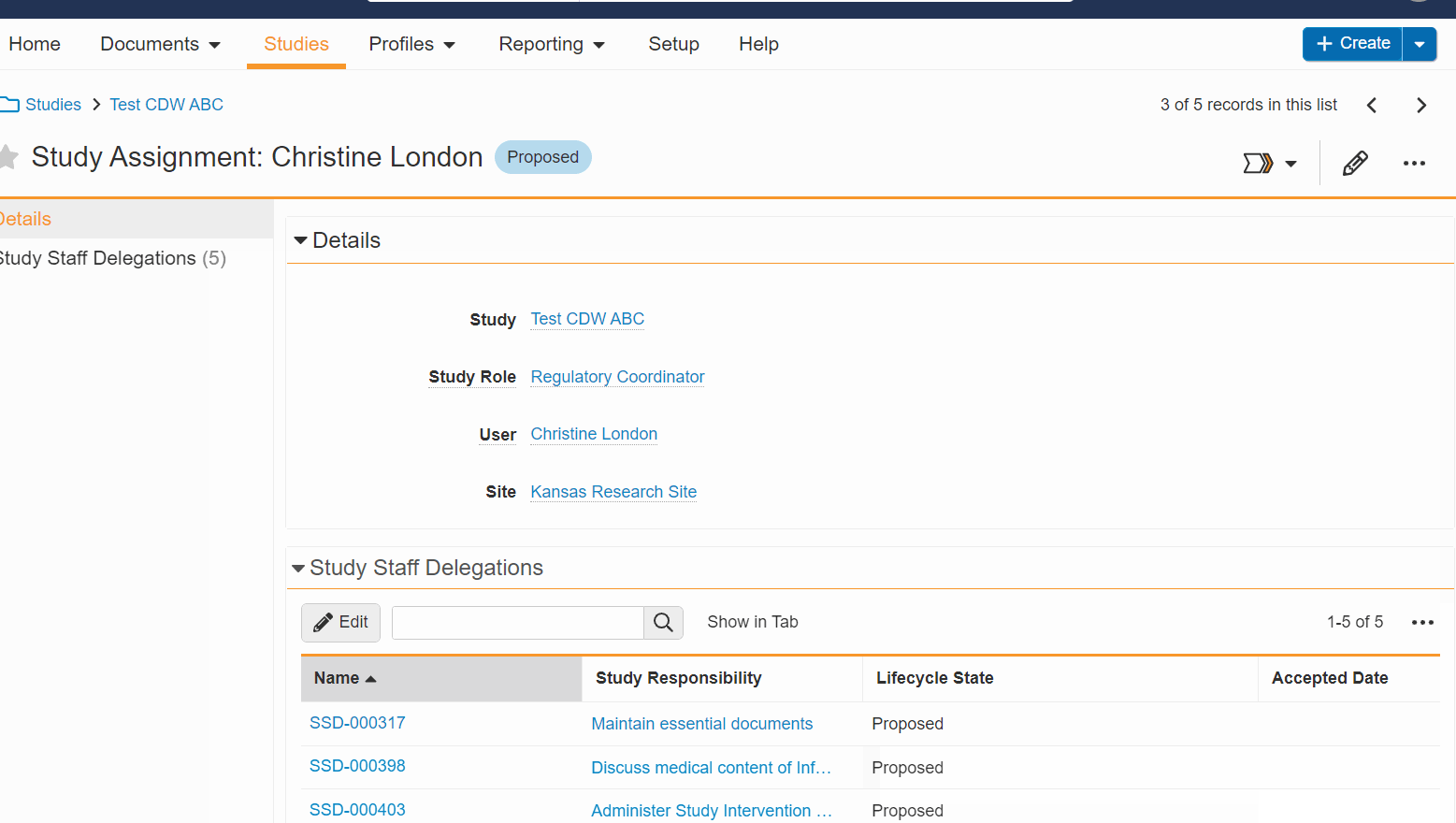
See the help for the Digital Delegation feature for more information.
New Read and Understand Workflows
This feature introduces three new Read and Understand workflows that replace the two existing Training workflows. The new workflows allow any document on the Draft to Final, Draft to Approved for Use, or Draft to Current lifecycles to be sent to any members of the study for them to acknowledge that they have read and understood a document.
User Administration for Large Research Organizations
This feature enhances the process of creating new users for large research organizations. When a Research Organization Administrator user in a Research Organization with more than three Sites creates a new user, all of the site-level system roles default to No Access.
New Study Role
With this feature, you can now assign study team members a study role of Other Non-Investigator when assigning them to a study. Those assigned this role are eligible to participate in study document or Digital Delegation workflows.
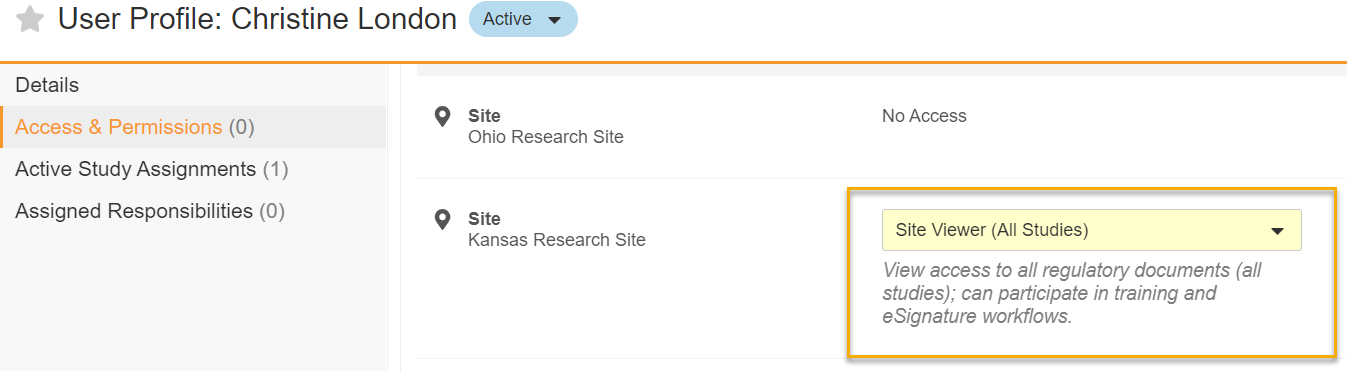
Site Viewer System Role
With this feature, the Site Viewer (All Studies) site-level system role is available. Site staff with this role can access (but not edit) study information and documents as well as participate in relevant workflows.
New Document Type
You can now create documents under the document type Source Data Agreement. This document type can be exchanged with a sponsor or CRO’s vault on a Connected Study.
Connected Studies
New Exchangeable Document Types on Connected Studies
This feature adds Ancillary Committee Submission and Source Data Agreement to the list of available document types that can be exchanged with a sponsor or CRO on a Connected Study.
Connected Study Type on Study
A new Connected Study Type field is displayed on Study records that indicates which Connected Study components are enabled by the sponsor for that study, including Document Exchange, Safety Distribution, Veeva eConsent, or Veeva ePRO.

Veeva eConsent
Support Microsoft Word Documents for eConsent Forms
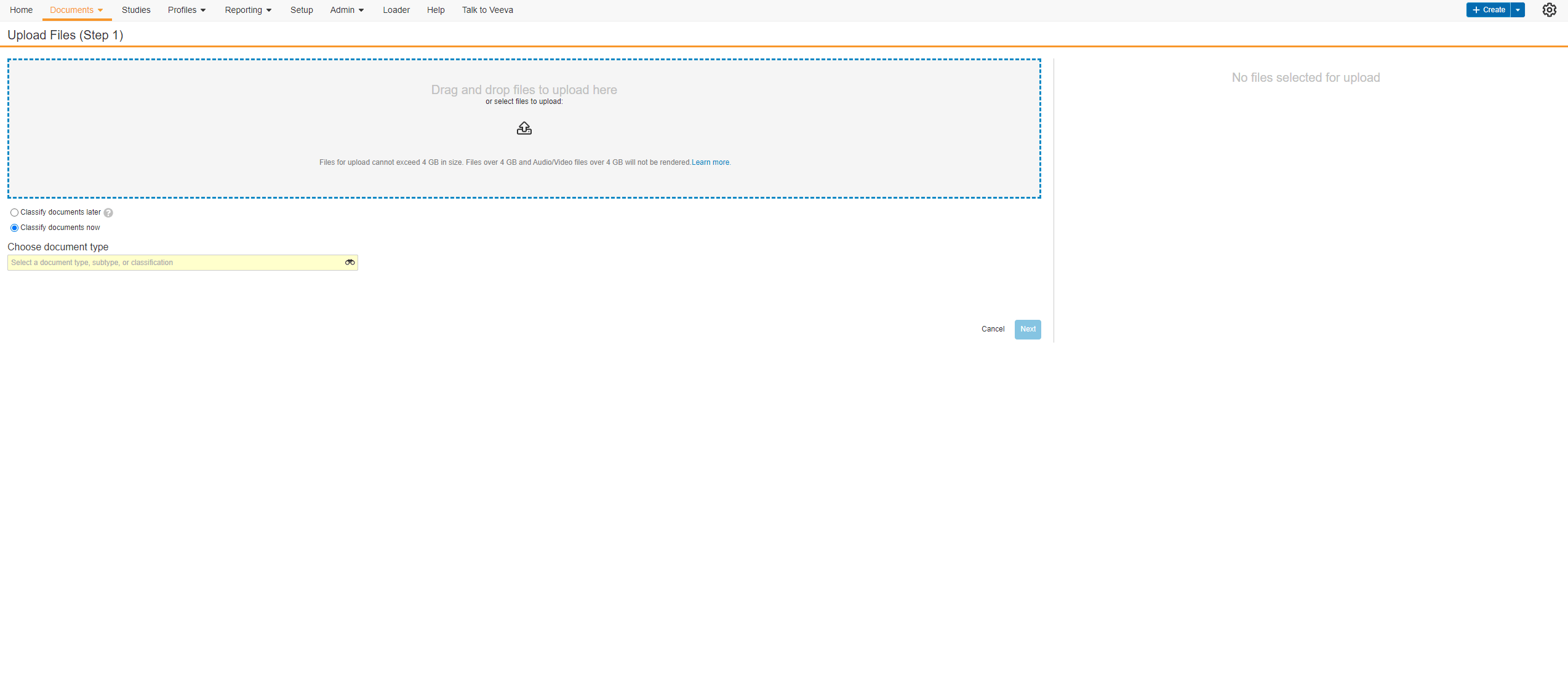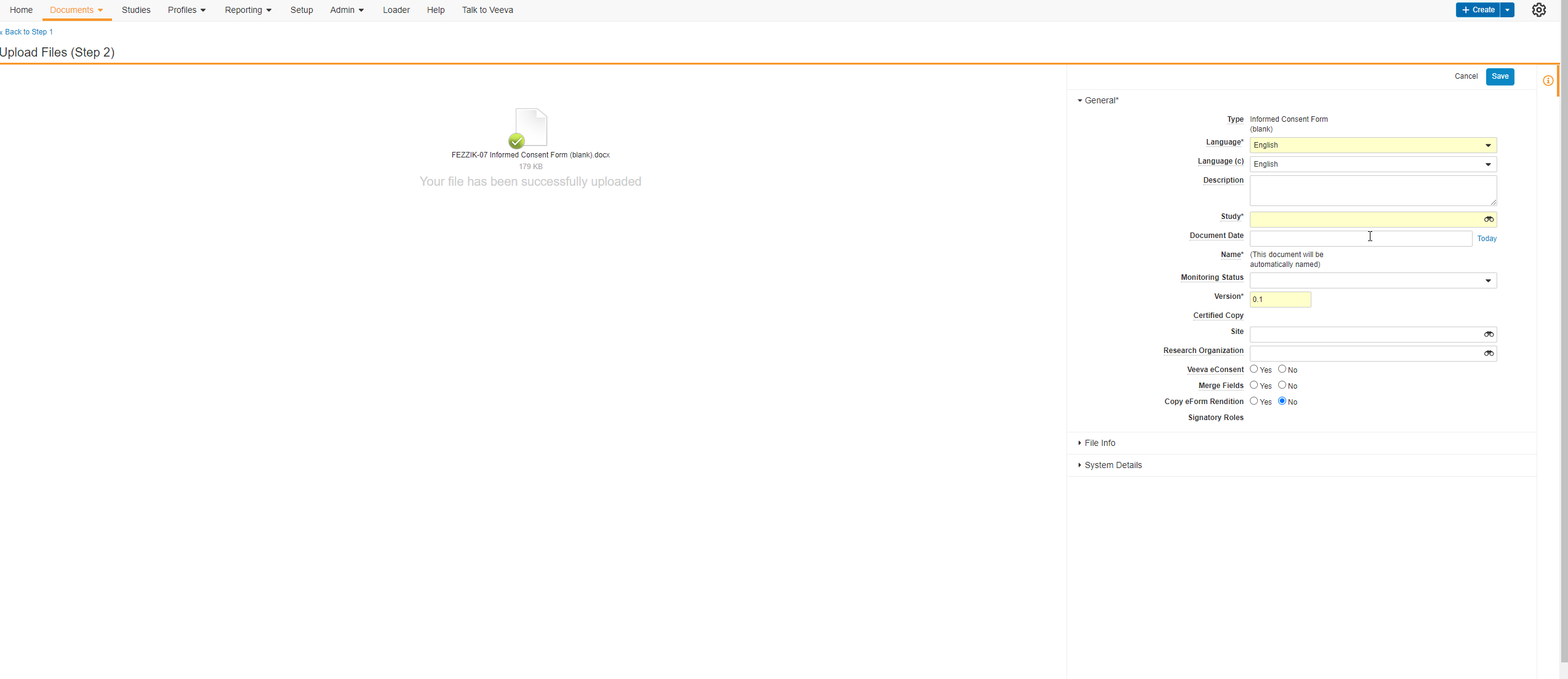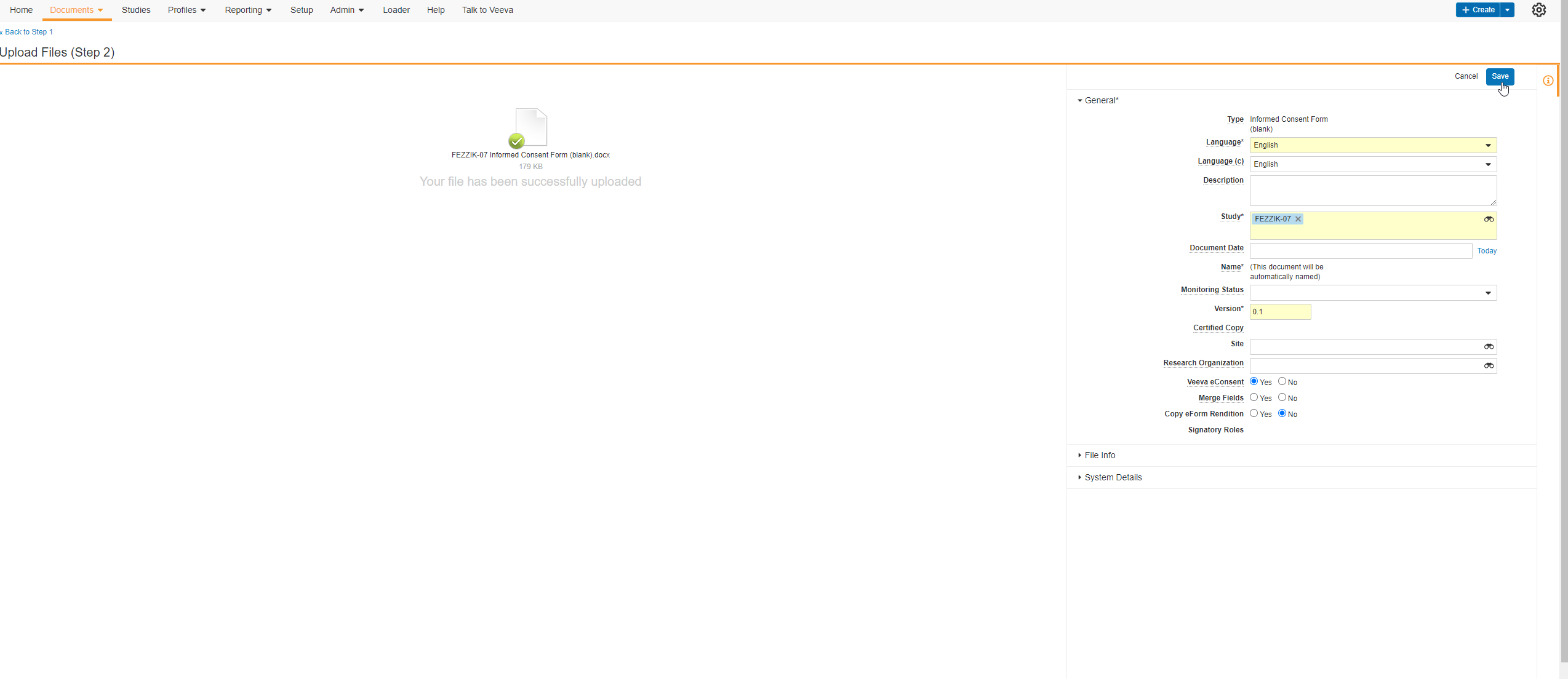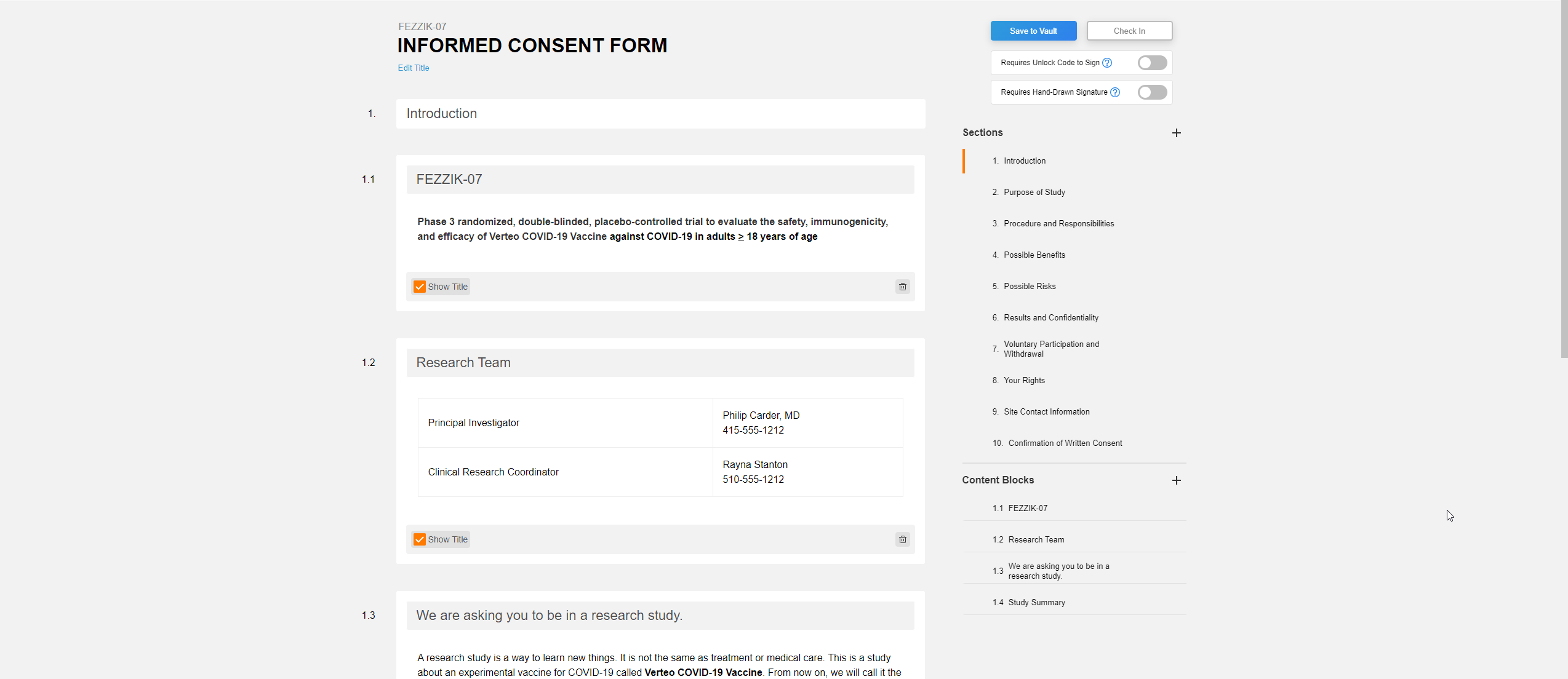
This feature allows users to create and use eConsent-compatible Microsoft Word™ documents in the Veeva eConsent editor. Users will be able to use the eConsent editor to manage their eConsent-specific features (such as signature blocks, images, or videos) while using Microsoft Word™ to create comments, track changes, and share revisions with various stakeholders. Users can upload their Microsoft Word™ files to SiteVault and integrate them into the eConsent editor for additional editing and can share the document with other users while it’s in a preview stage using a preview link.
The Veeva eConsent editor should be used to manage any eConsent-specific assets (such as signatures blocks, images, or videos) at the start of the consent form creation phase.
See Creating, Editing, Previewing, and Approving eConsent Forms for more information.
Veeva ePRO
Note Veeva ePRO must be configured by Veeva support in Vault Clinical Operations.
ePRO Connection
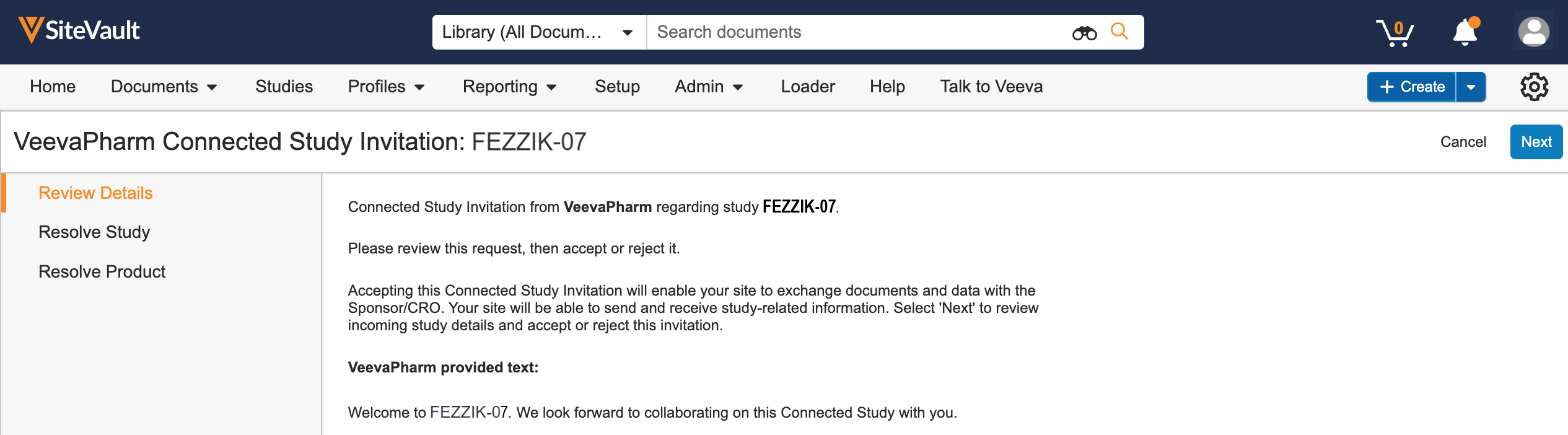
This feature allows sites to connect a study in SiteVault to a sponsor’s ePRO by entering a sponsor-provided ePRO code or by accepting a Connected Study Agreement.
Language Management
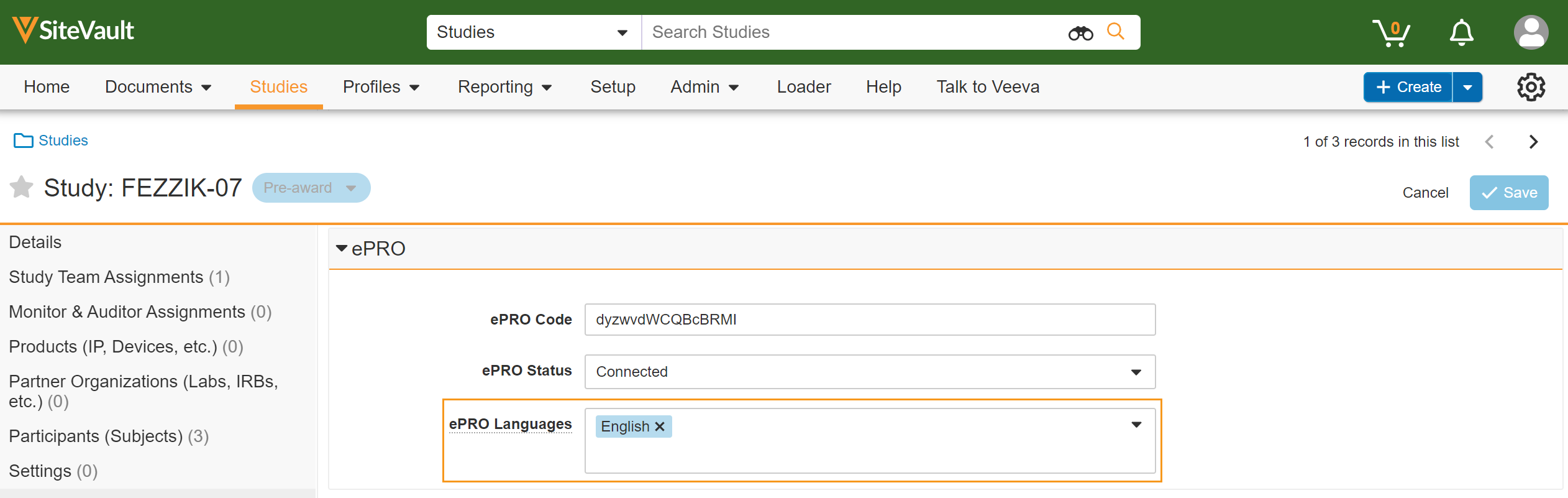
This feature allows sites to add and remove supported languages for a study’s ePRO in SiteVault. Users are prevented from adding languages that aren’t supported by the study or from removing languages that have already been approved by their IRB/EC.
ePRO Collection Document
This feature allows sites to download an ePRO collection document that contains all versions of a study’s ePRO, screenshots of all surveys in each language supported by the site, applicable notifications in each language supported by the site, and more. The ePRO collection document can be used to aid local IRB/EC approval. This document must be approved before participants can be enabled with ePRO.
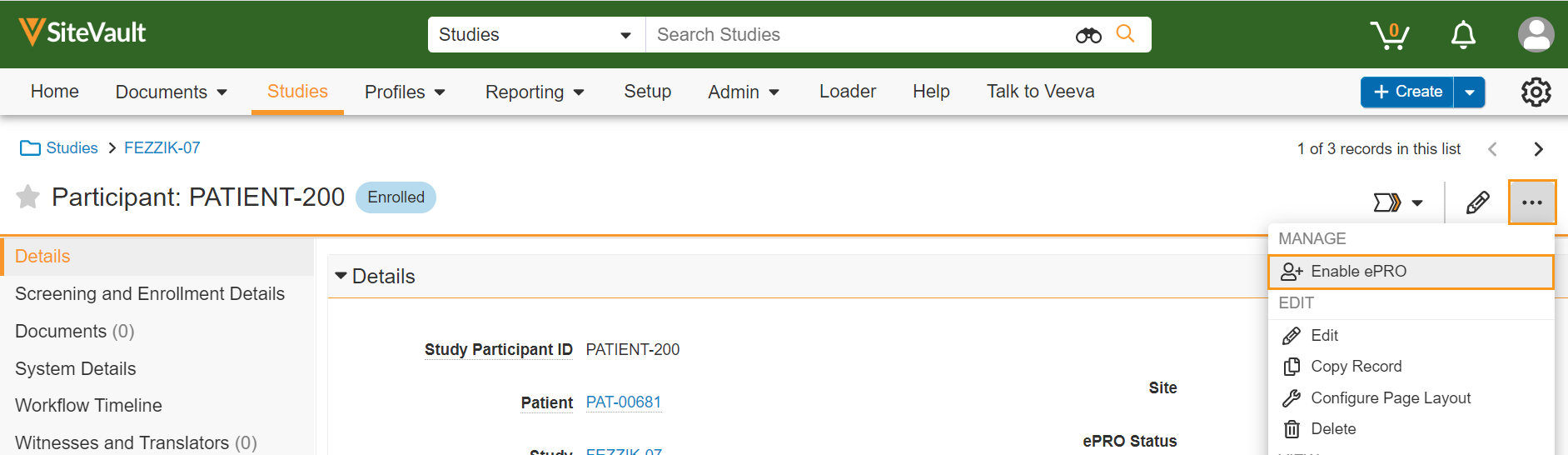
ePRO Enablement
This feature allows sites to enable and disable ePRO for participants when an approved ePRO collection document exists for a study.
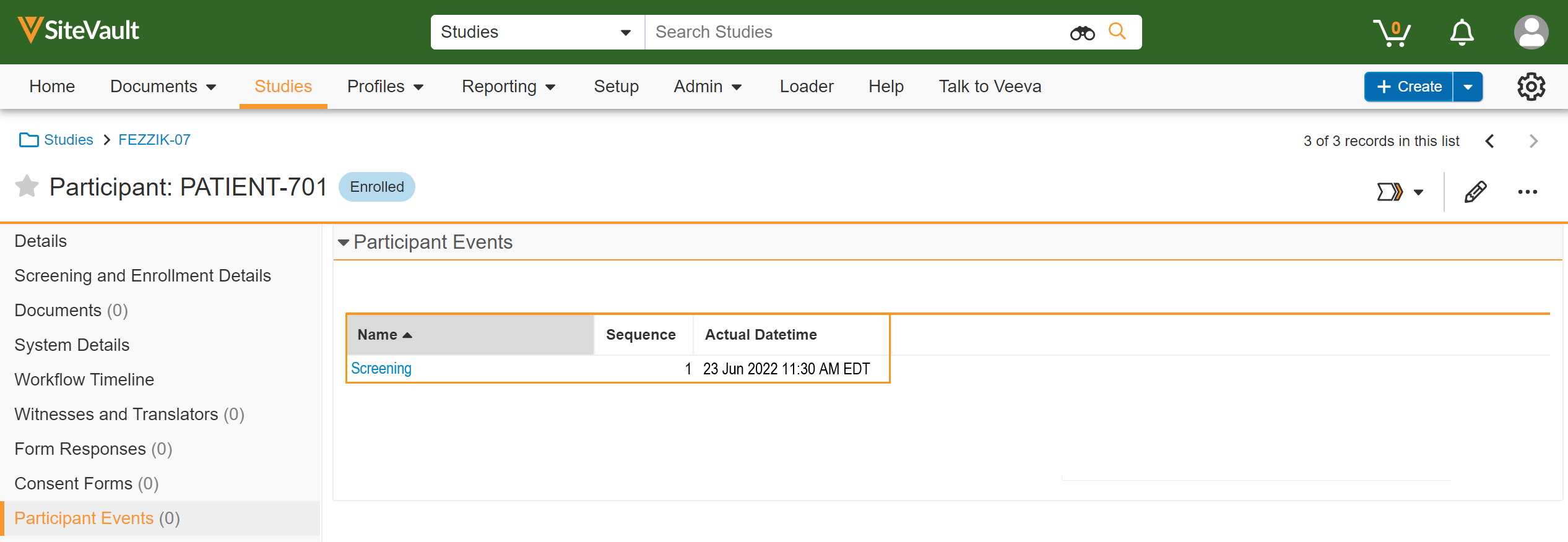
Participant Event Management
This feature allows sites to trigger schedules for a participant by adding, updating, or removing actual date/times for participant events.
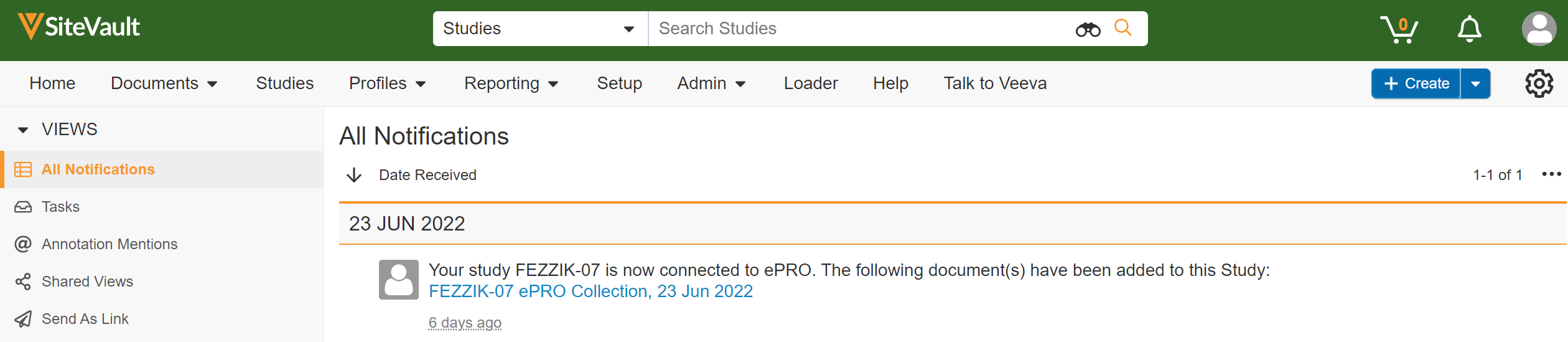
Site ePRO Notifications
This feature allows sites to receive ePRO-related notifications. Notifications may be received for successful or unsuccessful attempts to connect ePRO, update languages, approve ePRO collections, and more. Notifications may also be received when a participant has an upcoming survey to complete or has missed one or more surveys, if those notifications are configured for a study’s ePRO.
Site Data Export
This feature allows sites to export survey data, adherence data, and audit trail data for a study.