October 15, 2021 | Release Number: 21R2.0.06b
With this release, we’ve updated the look, feel, and usability of SiteVault to make your experience even more intuitive and enjoyable. See below for some of the key changes with this new UI.
Action Bar

A new Action Bar continuously learns how you work and displays your most frequently used actions.
- Workflow Actions Menu: Enables you to quickly start a workflow or make a state change, such as approving or finalizing a document or changing the state of a study participant record. Previously, these actions were located in the Actions menu.
- Most Frequently Used Actions: Your four most frequently used actions are displayed here. The actions that are displayed depend on the context. SiteVault learns the actions that you use most for a particular document type or record and displays those actions first.
- All Actions Menu: The All Actions menu is updated from the the gear icon (
 ) to an ellipses icon (). This menu contains all of the other (non-workflow) actions for a document or record (Create Draft, Make a Copy, and so on). As part of the UI refresh, anywhere the gear icon (or action wheel) previously appeared, it has been replaced with the ellipses icon.
) to an ellipses icon (). This menu contains all of the other (non-workflow) actions for a document or record (Create Draft, Make a Copy, and so on). As part of the UI refresh, anywhere the gear icon (or action wheel) previously appeared, it has been replaced with the ellipses icon.
Notifications
A new Notification Bell in the upper-right corner makes it easier for you to be aware of the documents, records, and tasks that need your attention. When you receive a notification, an orange notification dot is displayed on the icon. Select the icon to view your notifications, and select View All on the list to view all of your notifications on a new notifications page (this replaces the Notifications view that was previously available in the Home tab).
Doc Info Pane Updates
The Doc Info pane is now divided into the five panels below. In addition, you can expand or collapse the entire Doc Info pane or select and drag to resize the pane as needed.
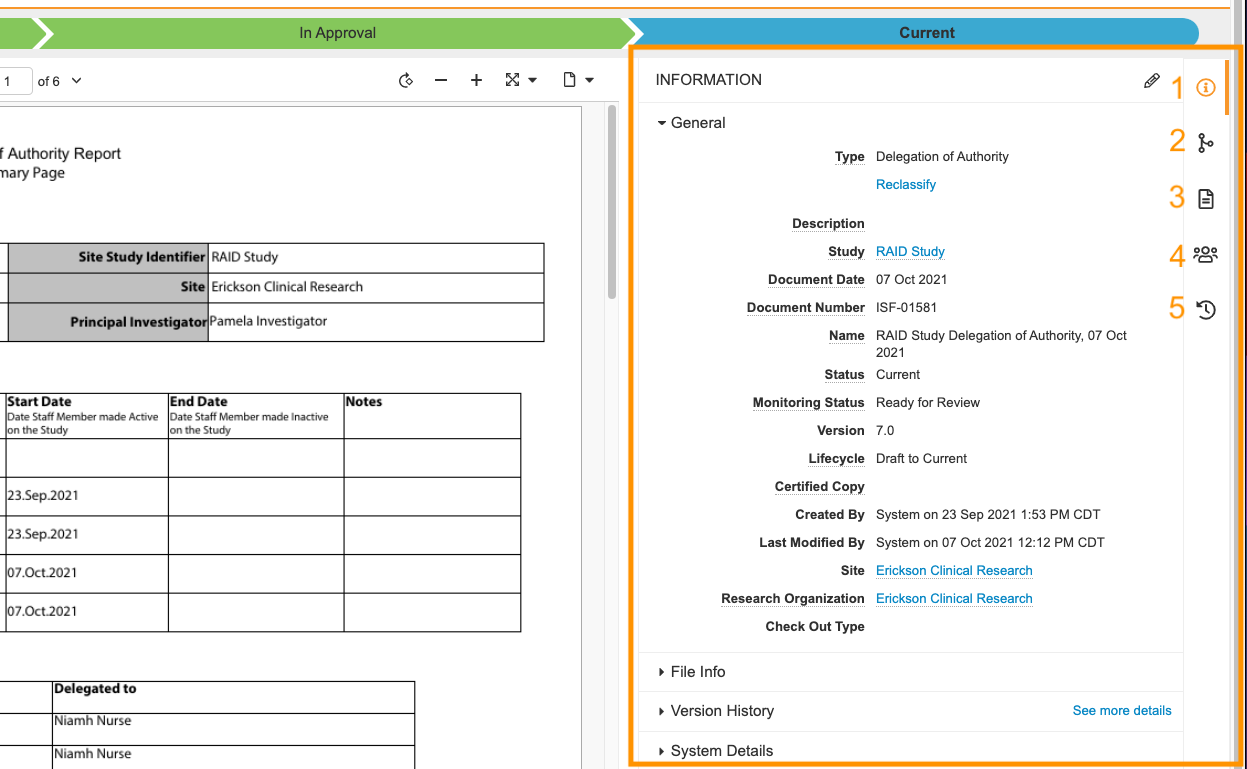
- Document Information
- Relationships
- Document Files
- Sharing Settings
- Timeline View
October 15, 2021 | Release Number: 21R2.0.06a
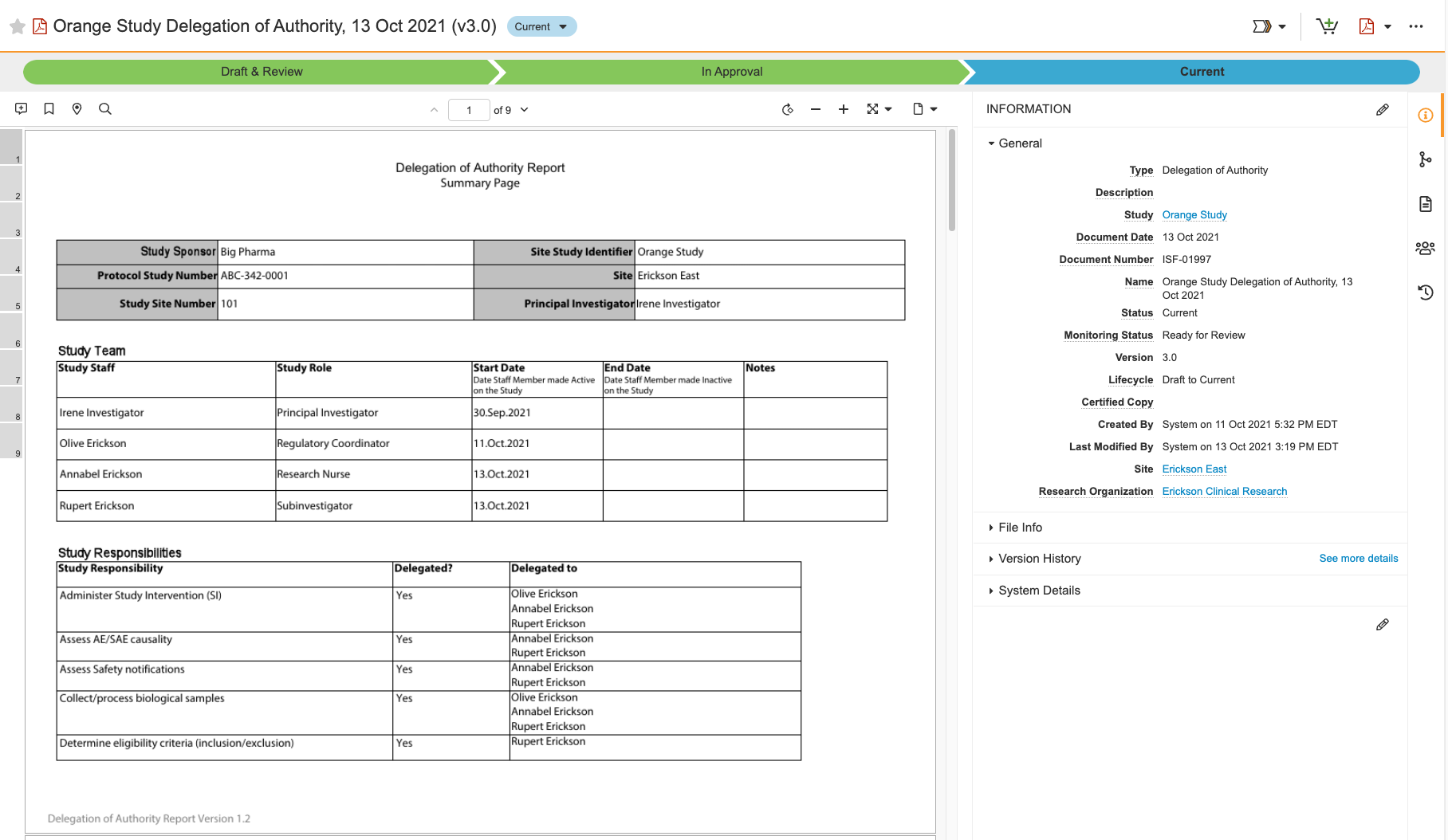
This release introduces the Digital Delegation feature. A Delegation of Authority (DoA) log provides a record of a principal investigator’s (PI) decisions to allow specific site staff members to perform one or more of the PI’s study responsibilities. Instead of having to manually manage a paper-based, handwritten DoA log, the Digital Delegation feature enables users at your site to delegate, accept, and approve responsibilities right in SiteVault, and SiteVault creates or updates the DoA report for you.
See the Overview of Digital Delegation page to learn more and to get started.
September 17, 2021 | Release Number: 21R2.0.04
Introducing System Roles and Add-on Permissions
This release introduces a new way to administer user access and permissions in SiteVault. The previous SiteVault user types (for example, Regulatory and Study Team) are now replaced with the following site-level system roles:
- Site Administrator: Can view and manage all information and documents, adjust settings, and add new users.
- Site Staff: For all nonadministrative research staff. For assigned studies, can create study participants and send them eConsent documents.
- Site External: Read-only access. Unable to view study information until added to the study.
In addition, this release introduces permissions at the level of your research organization with the following system roles:
- Research Organization Administrator: Has full access to all data and functionality. Can create and manage user accounts at the research organization level (across multiple sites, if applicable).
- Research Organization Staff: For all nonadministrative research staff. Provides view access to research organization-level information.
- Research Organization External: For monitors, CRAs, and auditors. No access to any research organization-level data or documents.
These new system roles provide users with permissions specific to your research organization and specific to each of your sites. In addition, you can add additional permissions to a user’s site- or research organization-level system role. For example, you can provide a user with permissions to manage patient information for all studies at one site or all sites in your research organization.
At the research organization level, you can extend permissions for certain users to manage patient and recruiting information across all sites by giving those users the patients & recruiting add-on permission. At the site level, you can extend permissions for certain users to manage any or all of the following areas for that site: patients and recruiting, budgets and contracts, and profile information.
Research Organization Administrators and Site Administrators can create users and manage system roles and add-on permissions.
Migrating Your Current Data
As part of this release, SiteVault automatically migrates your existing users to these new system roles. The following data migration considerations apply:
- Users with the Regulatory user type are updated to the new Site Administrator system role
- Users who had the Regulatory user type for all sites in their research organization are also given the Research Organization Administrator system role
- Users who had the Regulatory user type for some but not all sites are given the Research Organization Staff role
- Users with the Study Team user type are updated to the new Site Staff system role
- Users with the External user type are updated to the new Research Organization External and Site External system roles
August 19, 2021 | Release Number: 21R2.0.03
Patient Phone Numbers and Email Addresses
SiteVault now checks whether the phone number and email address are formatted correctly when you add or update them for a patient. In addition, when entering a phone number for a patient, you now select the country from a list before entering the area code and phone number, then SiteVault automatically updates the number to the correct format for you.
Note Only phone numbers and email addresses added or updated after this release are validated. If you’re consenting a participant using Veeva eConsent and the phone number or email address was entered incorrectly before this release, you’ll need to correct the information on the patient record, cancel the eConsent forms you sent, and resend the eConsent forms. See Troubleshooting Veeva eConsent and MyVeeva for Patients for more information.
August 11, 2021 | Release Number: 21R2.0.02
Veeva eConsent
In-Person Consent
This feature makes it easier for sites to use Veeva eConsent in situations where it’s difficult for the patient to access their email account, such as when consenting in-office or when they do not have their own device. Site staff can create an in-person consent QR code in SiteVault, and the participant can scan the code or use the onscreen web address to access MyVeeva for Patients. When the participant completes all their documents, they’re logged out automatically after 30 seconds of inactivity or if the browser is closed.
See the Start the Consent Process page for more information.
Participant Preview
With this feature, you can view and share a preview of the patient experience of an eConsent form in MyVeeva for Patients. This enables you to more easily obtain EC/IRB approval of the eConsent form. When a preview is shared with an external user, the user can view the preview without a SiteVault or MyVeeva for Patients account, and the preview links expire after 30 days.
See the Previewing and Sharing an eConsent Form section on the Managing eConsent Forms page for more information.
Form Responses
For any questions, response options, and signatures that are included on your eConsent form, you can view and report on the responses collected from participants in MyVeeva for Patients during the eConsent process. The responses and signatures are recorded in a new Form Responses section on a study participant’s record.
See the [Viewing and Reporting On eConsent Form Responses](/gr/sitevault/econsent/view-responses/ page for more information.
August 6, 2021
Veeva eConsent
Veeva eConsent is now generally available. You can use Veeva eConsent with MyVeeva for Patients to consent study participants electronically.
See the following help pages and release notes for more information:
- Overview of Veeva eConsent
- MyVeeva for Patients Help on the MyVeeva for Patients Help Website
- MyVeeva for Patients Release Notes