Note Veeva ePRO must be configured by Veeva support in Vault Clinical Operations. SiteVault ePRO functionality is available to only sites conducting studies with sponsors.
About the Release
Important Dates
| Date | Event | Description |
|---|---|---|
| July 11 | Pre-Release | The release is deployed to the MyVeeva for Patients pre-release environment and available for customers to explore from pre-release Vaults. |
| August 5 | General Release | The release is deployed and generally available. The Digital Trials Platform validation binder will be available to customers in VeevaDocs for MyVeeva for Patients, SiteVault, Veeva eConsent, and Veeva ePRO at this time. |
Release Information and Impacts
| Resource | Description | Publication History |
|---|---|---|
| MyVeeva for Patients 22R2 Release Impact Assessment |
The release impact assessment analyzes the regulatory, configuration, and user impacts of the MyVeeva for Patients, Veeva eConsent, and Veeva ePRO features in this release. |
Updated July 25 |
| What's New |
The What's New information provides detailed explanations of the new features for MyVeeva for Patients, Veeva eConsent, and Veeva ePRO. |
Available July 5 |
| Fixed Issues |
The Fixed Issues list documents issues that affected previous versions or the pre-release and are fixed in this general release. |
Available July 25 |
| Known Issues |
The MyVeeva for Patients Known Issues page lists issues that affect this release or previous ones and aren't fixed yet. |
Available July 25 |
| Maintenance Releases |
The MyVeeva for Patients Maintenance Releases page lists releases that fix issues affecting the general release. |
Available with the first maintenance release |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new features across the Digital Trials Platform:
|
See links |
What’s New
Veeva eConsent
Word-Based eConsent
This feature allows users to create and use eConsent-compatible Microsoft Word™ documents in the Veeva eConsent editor. Users will be able to use the eConsent editor to manage their eConsent-specific features (such as signature blocks, images, or videos) while using Microsoft Word™ to create comments, track changes, and share revisions with various stakeholders. Users can upload their Microsoft Word™ files to Vault and integrate them into the eConsent editor for additional editing and can share the document with other users while it’s in a preview stage using a preview link.
The Veeva eConsent editor should be used to manage any eConsent-specific assets (such as signatures blocks, images, or videos) at the start of the consent form creation phase.
For more information, see the following help topics:
- Using the Veeva eConsent Editor in Clinical Operations Help
- Creating, Editing, Previewing, and Approving eConsent Forms in the SiteVault Help
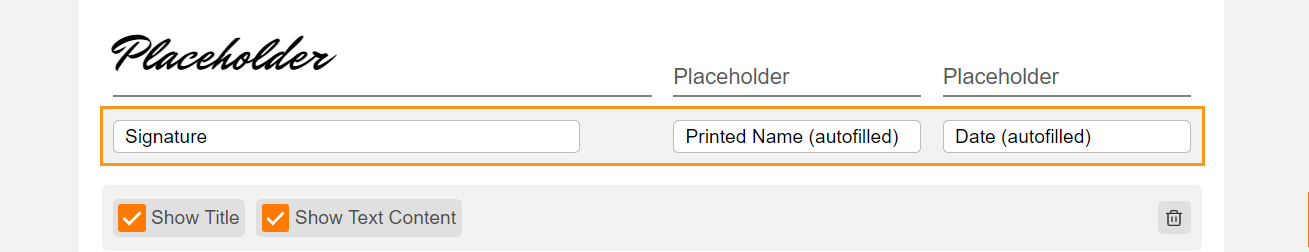
Edit Signature Line Labels
This feature allows users to edit the labels of the Signature, Printed Name, and Date fields on eConsent form signature blocks. Users can edit each label with a maximum character limit of 50. If none of the label fields on new eConsent forms are edited by the user, the text in each label field defaults to English. The label fields on existing eConsent forms will continue to use the participant’s preferred language setting in MyVeeva for Patients.
The Veeva eConsent editor should be used to edit and manage the label fields on signature blocks. Users may experience issues if they edit the labels using Microsoft Word™.
For more information, see the following help topics:
- Using the Veeva eConsent Editor in Clinical Operations Help
- Creating, Editing, Previewing, and Approving eConsent Forms in the SiteVault Help
Veeva ePRO
Veeva ePRO in Clinical Operations
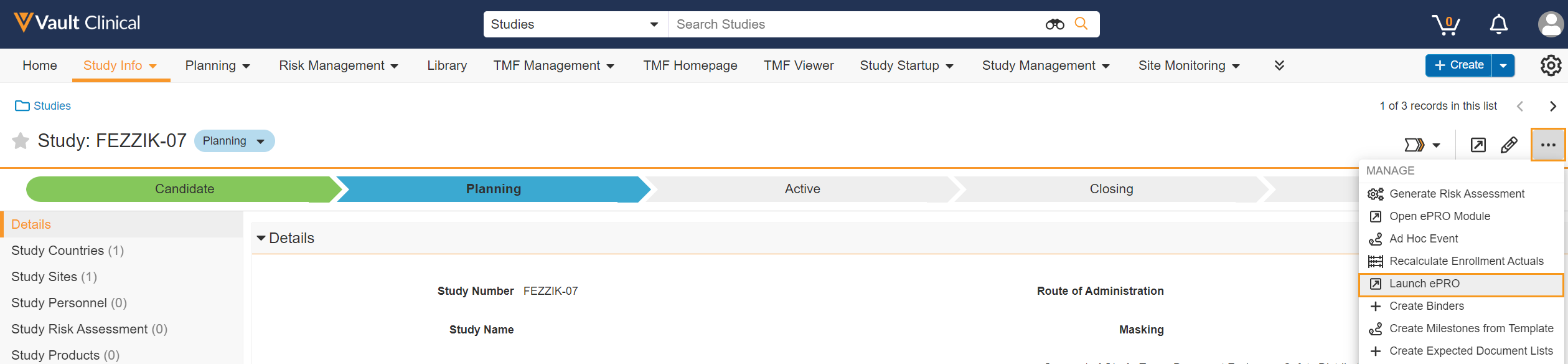
Module Security
This feature allows sponsors with the proper permissions to authenticate into the ePRO module for a study through a Clinical Operations Vault. The timeout length for the ePRO module will be the same length of time that’s afforded to them in the Clinical Operations Vault.
See the Navigating to the Veeva ePRO Module section in Clinical Operations Help for more information.
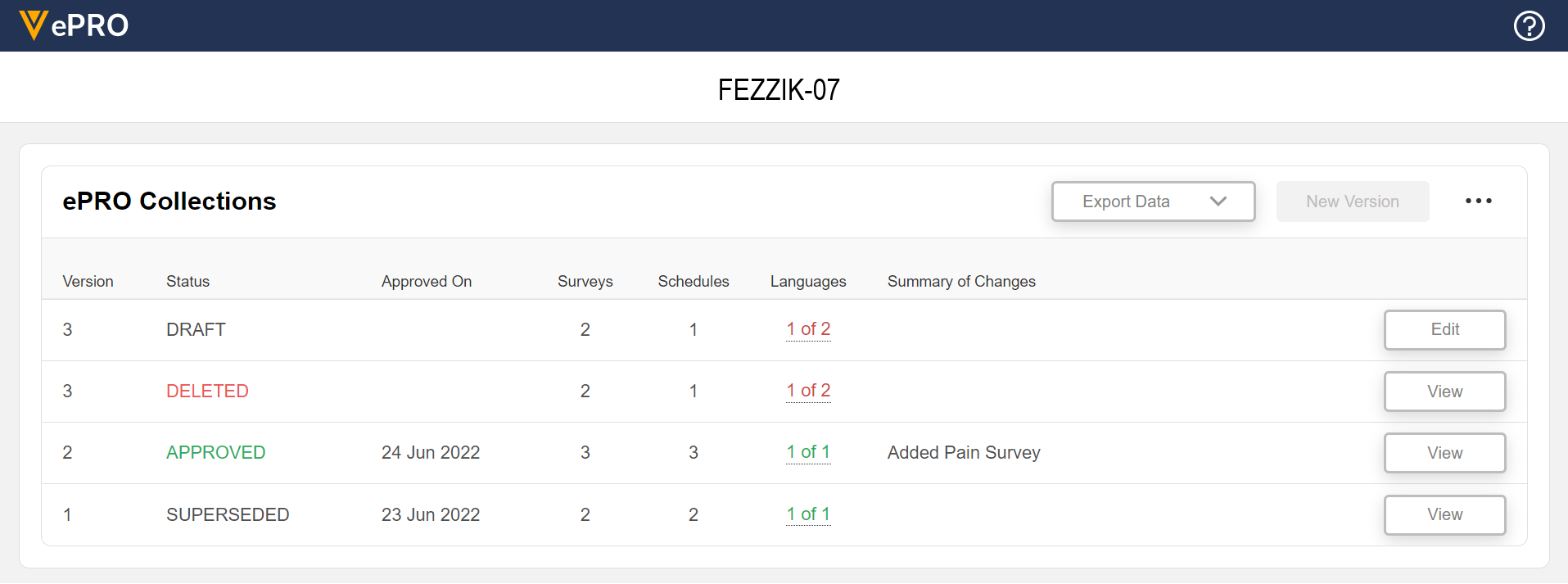
Collection Management
This feature allows sponsors to create, manage, and delete ePRO collections for a study. An ePRO collection is the collection of surveys, schedules, notifications, languages, translations, and events for a study’s ePRO. ePRO collections can be upversioned and approved as needed and can include a summary of changes between versions.
For more information, see the following help topics in Clinical Operations Help:
- Creating an ePRO Collection
- Viewing All ePRO Collections in a Study
- Approving an ePRO Collection
- Upversioning an ePRO Collection
- Deleting an ePRO Collection
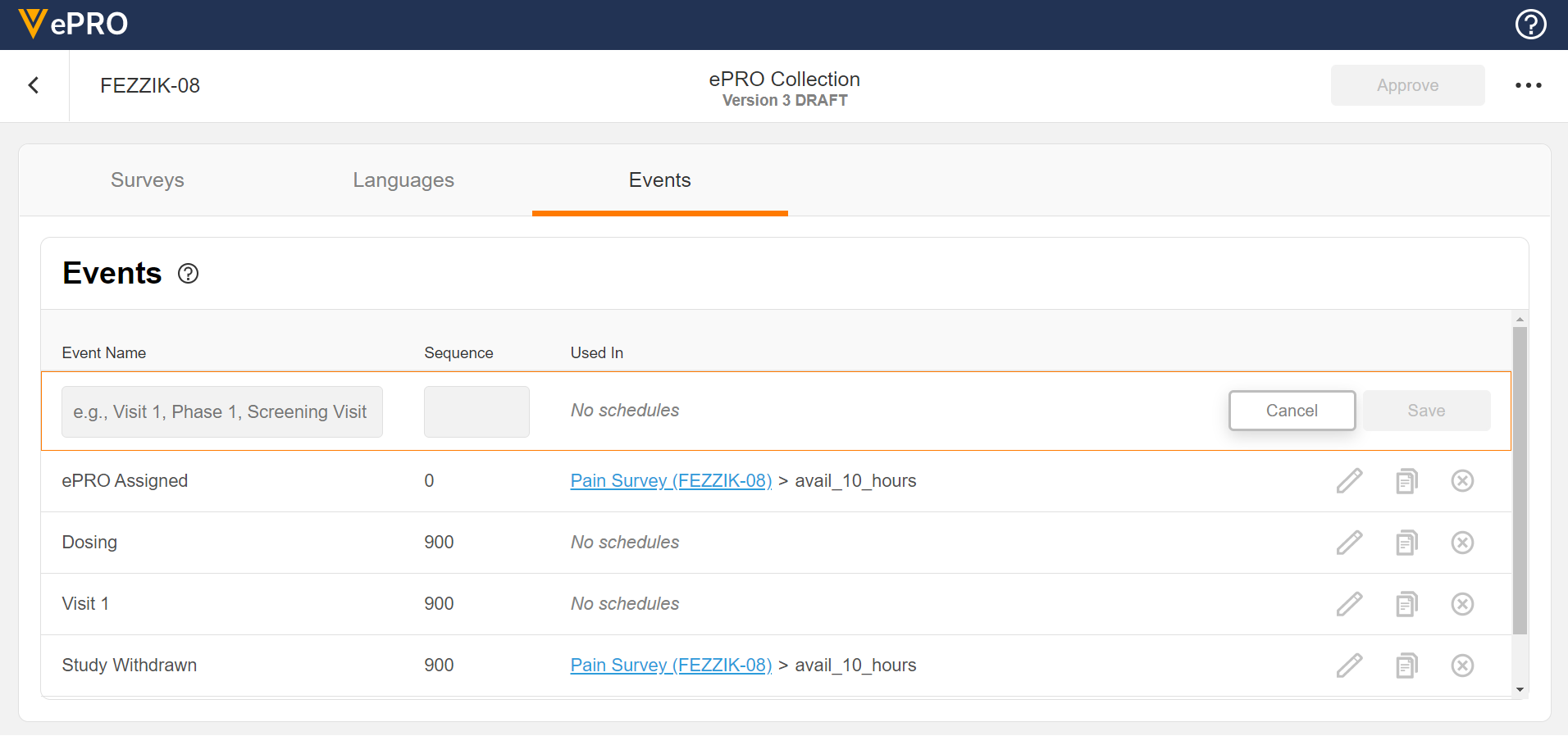
Event Configuration
This feature allows sponsors to create, edit, and delete events per the schedule of events in a protocol. Events can be named and sequenced, and an event’s unique identifier can be used to create schedules.
See the Managing Events page in Clinical Operations Help for more information.
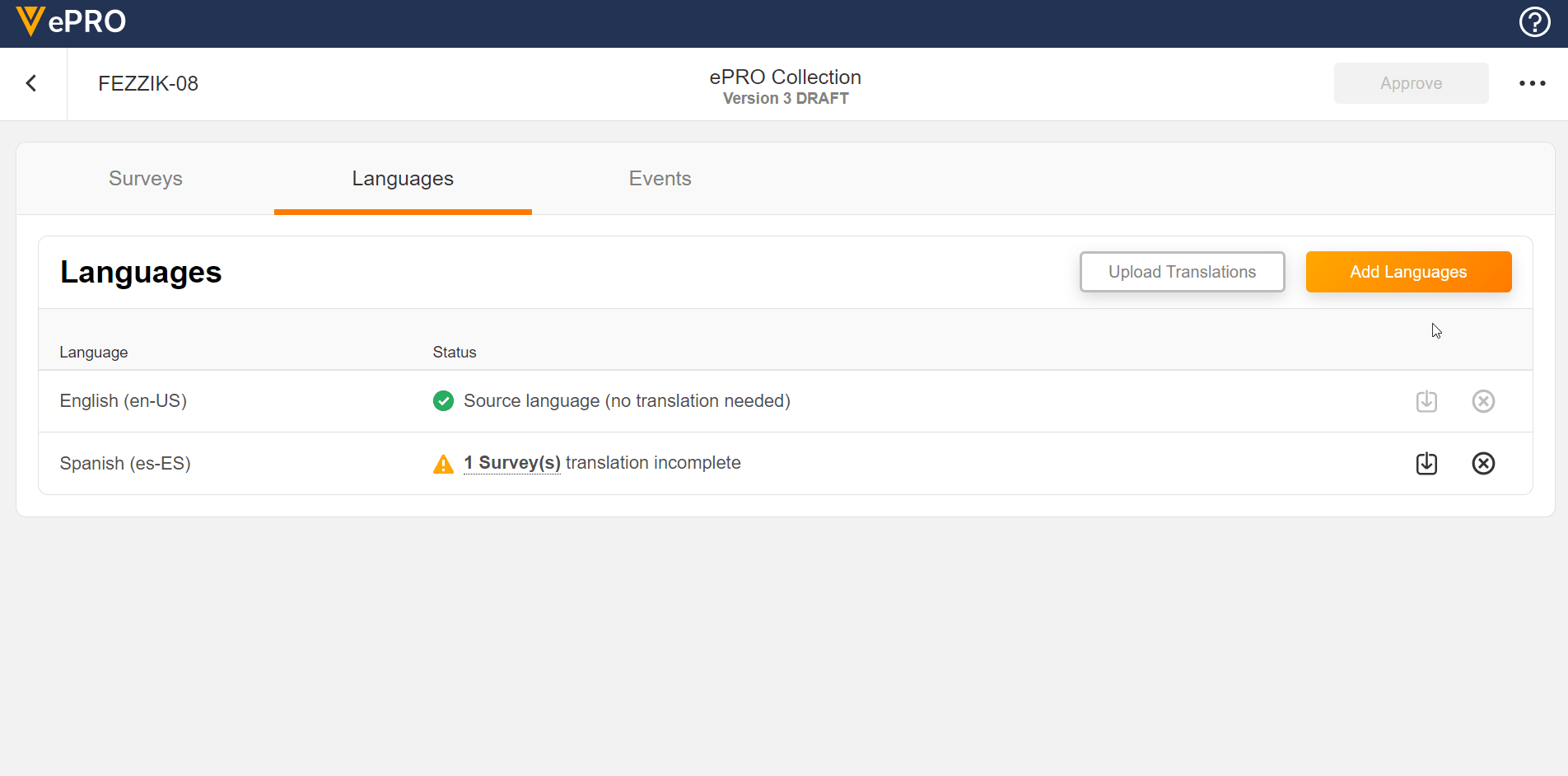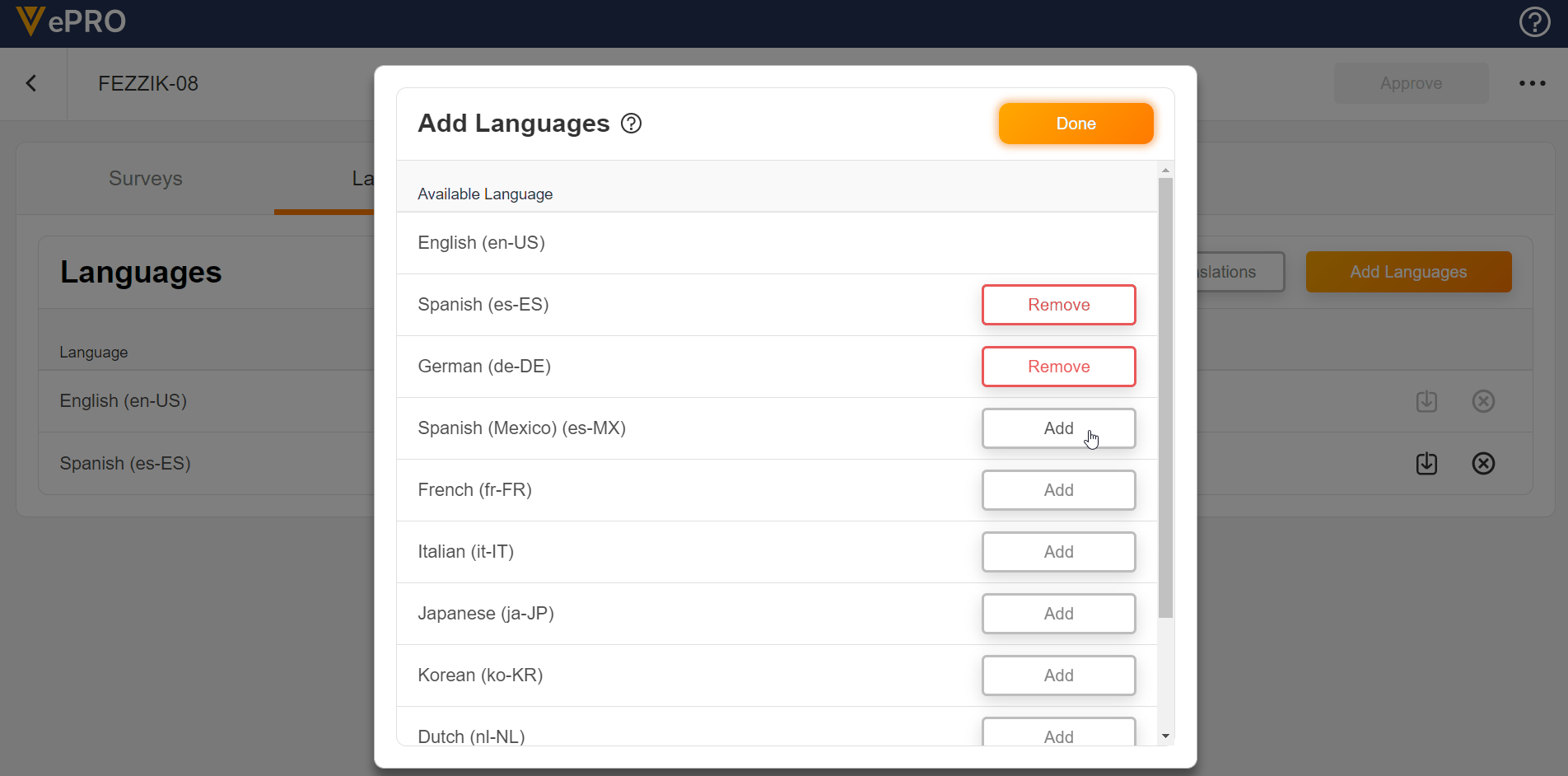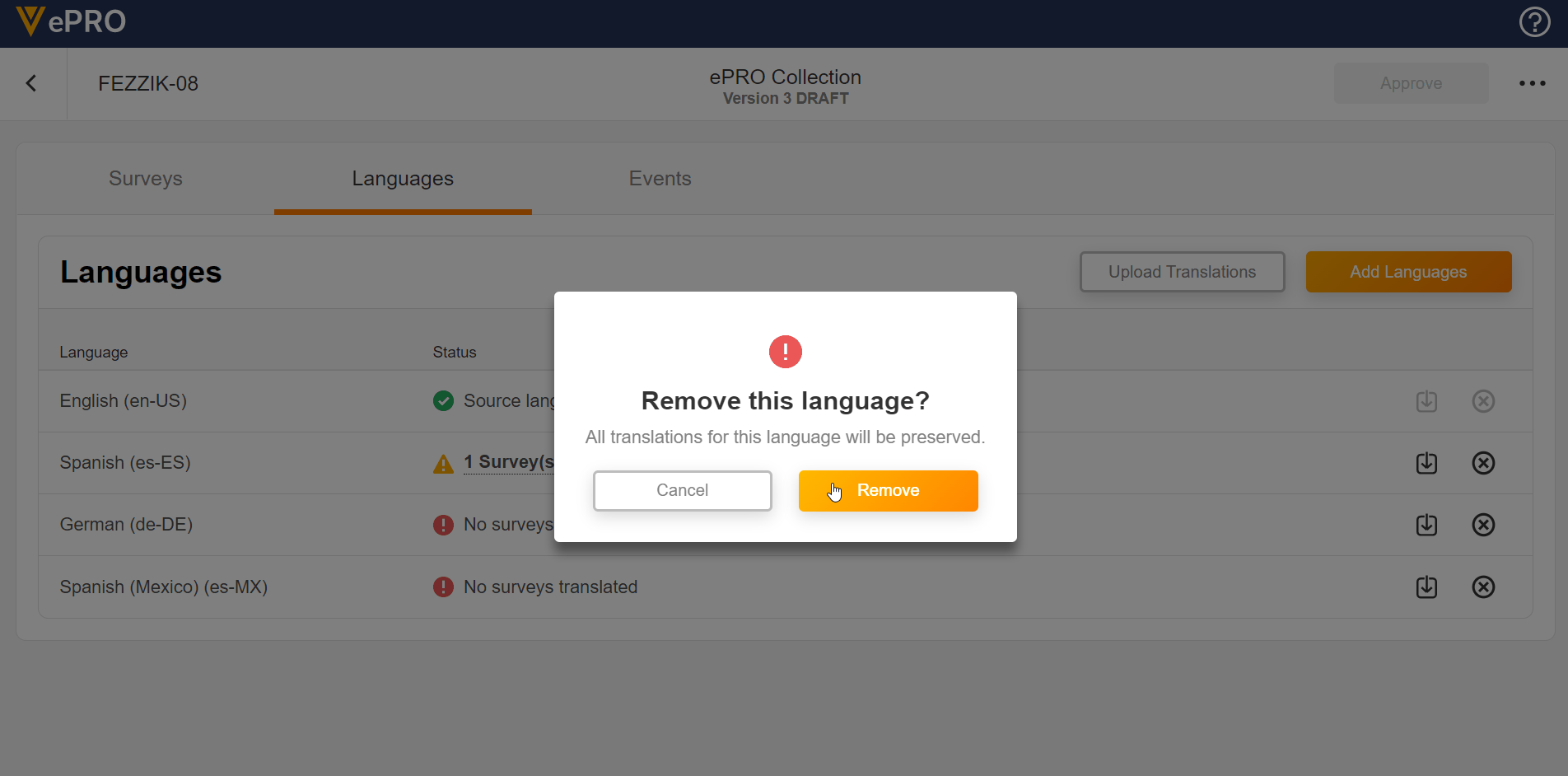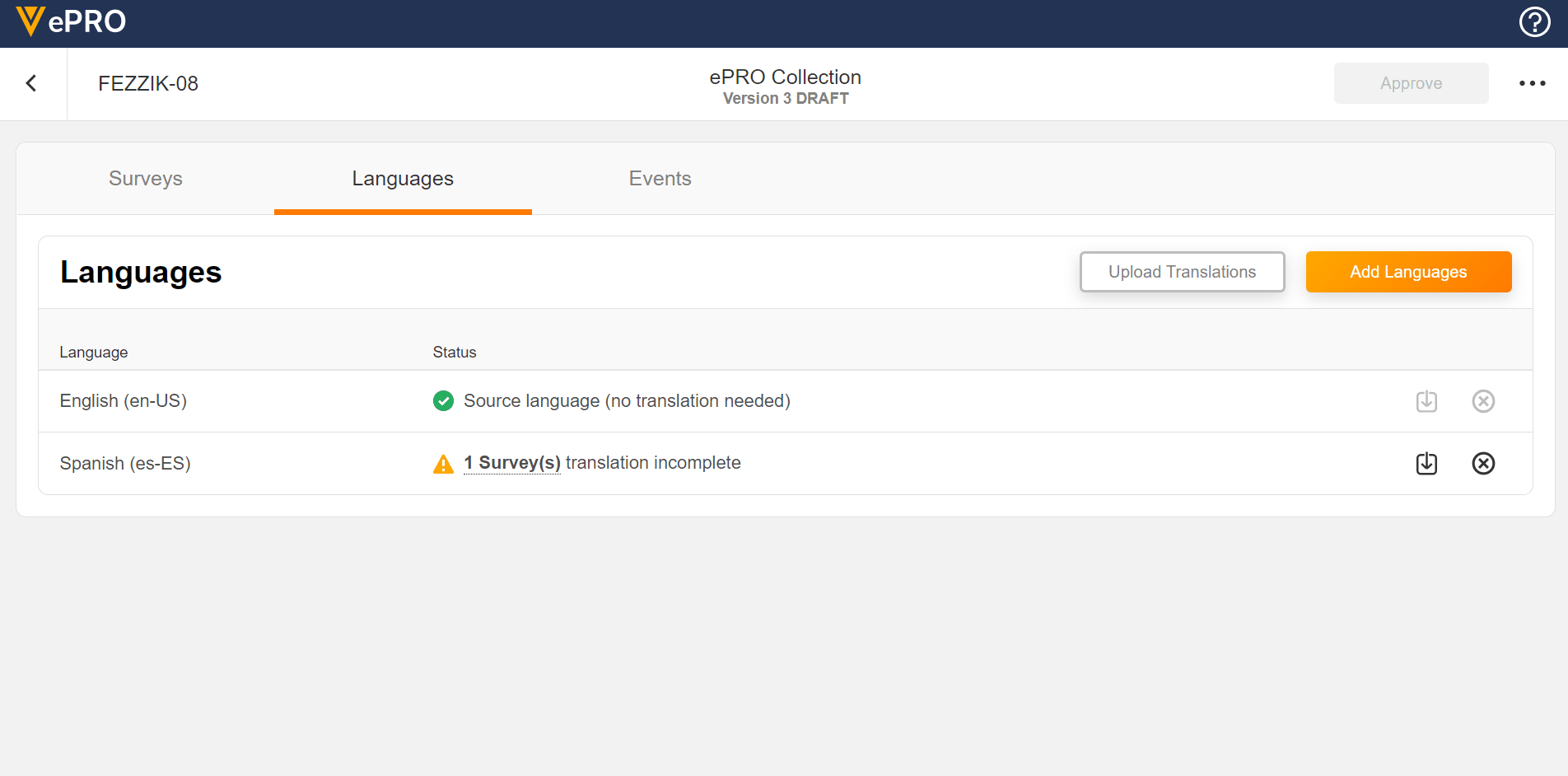
Language Configuration
This feature allows sponsors to add and remove languages for use in a study’s ePRO. The untranslated text for surveys can be exported and provided to a translation vendor. The returned translations for survey text can be uploaded for any added language.
See the Managing ePRO Languages and Translations page in Clinical Operations Help for more information.
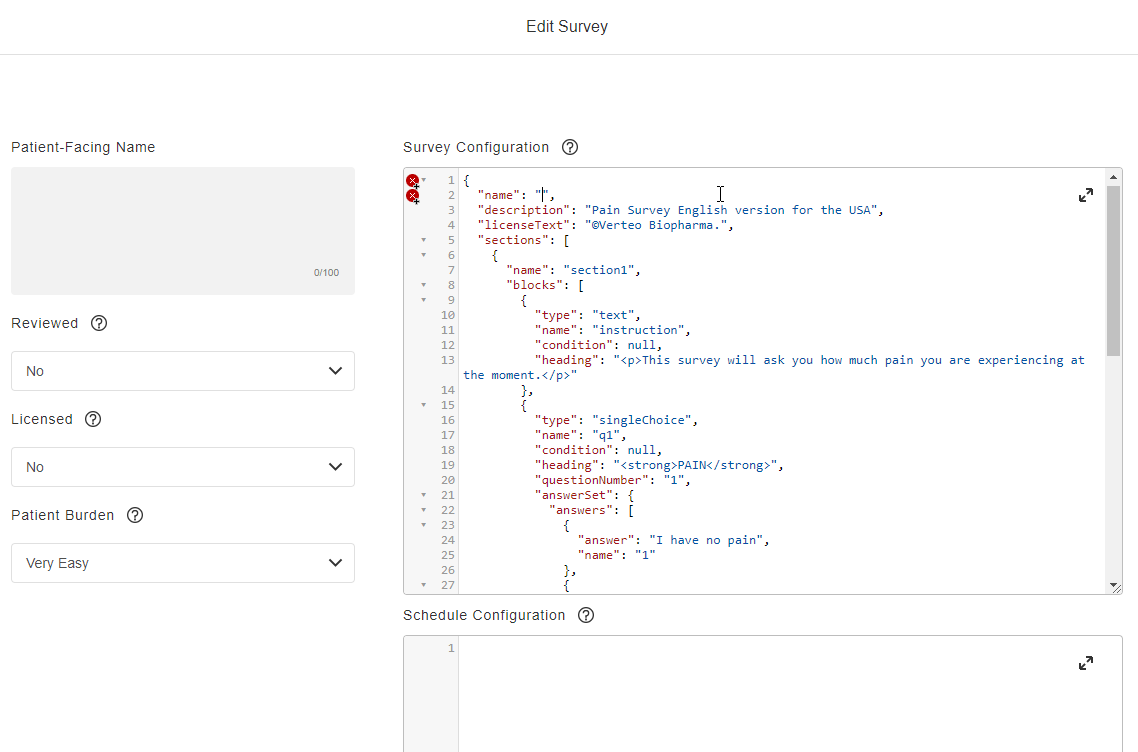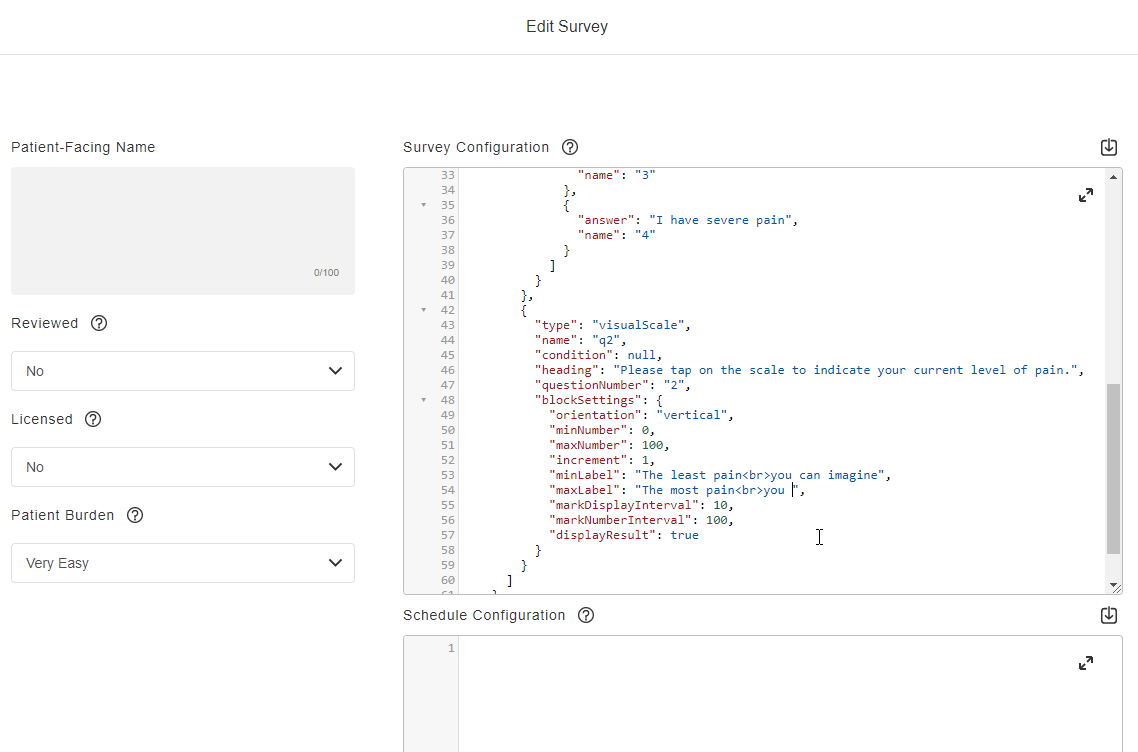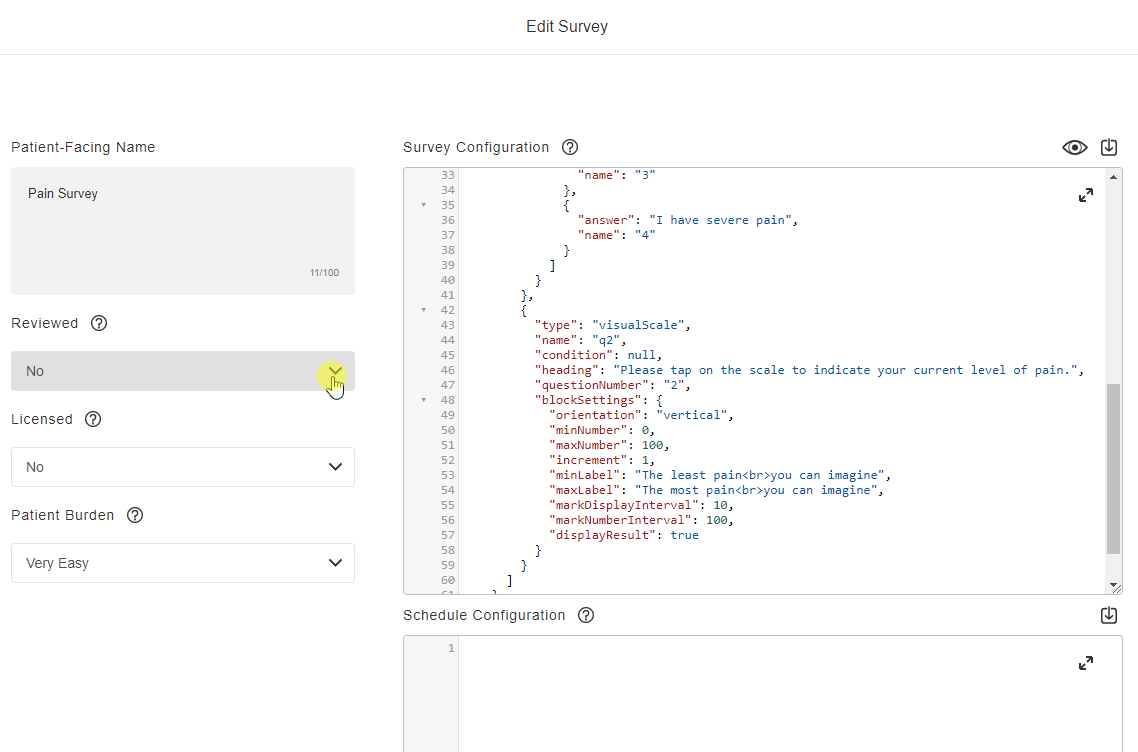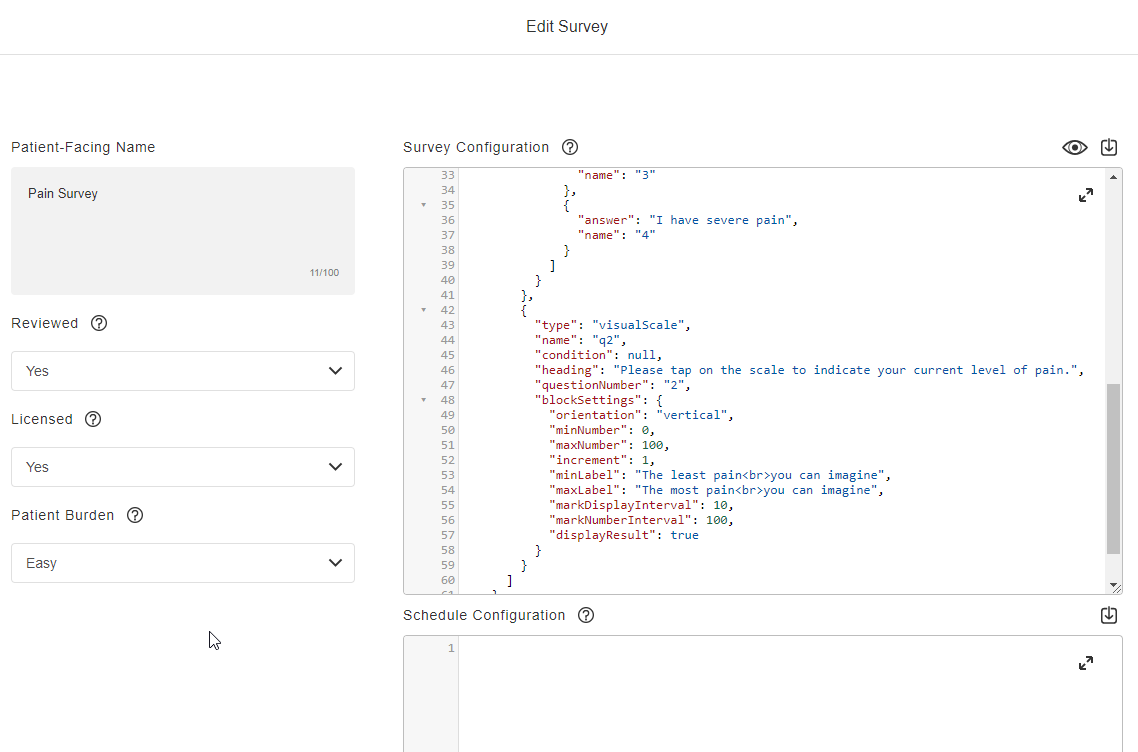
Survey Configuration
This feature allows sponsors to create surveys using JSON. Single-choice, visual analog scale, and numeric rating scale question types are supported, and a text block control allows for instructions to be displayed for a survey. Rich text styles such as bold, italic, and underline are supported in addition to breakline and paragraph tags. Users can define a patient-facing name for surveys and see a preview of the survey while creating it. License holder statuses for licensed instruments can be tracked.
See the Configuring Surveys page in Clinical Operations Help for more information.
Schedule Configuration
This feature allows sponsors to create schedules for a survey. Users can choose which events start or end a schedule for participants. Events can be configured to be available immediately, at a specified date/time, or for a specified period of time after the start event. Users can also create ad hoc schedules for surveys that make them available to participants on an as-needed basis.
See the Configuring Schedules and Notifications page in Clinical Operations Help for more information.
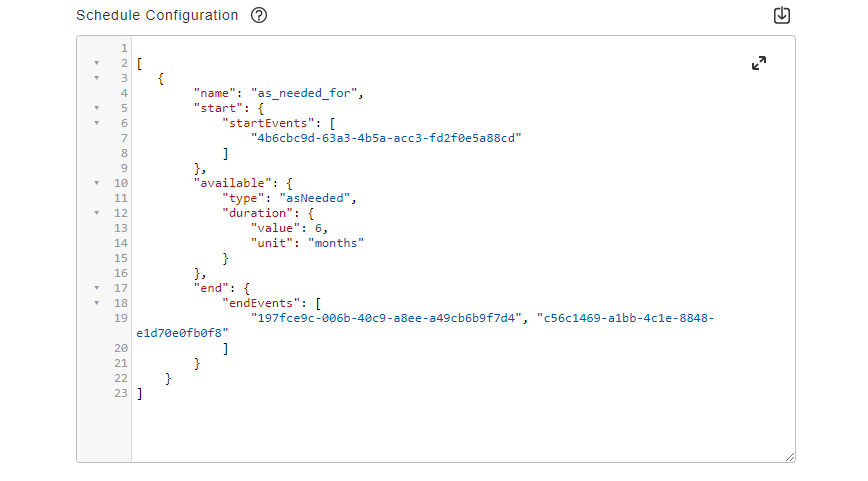
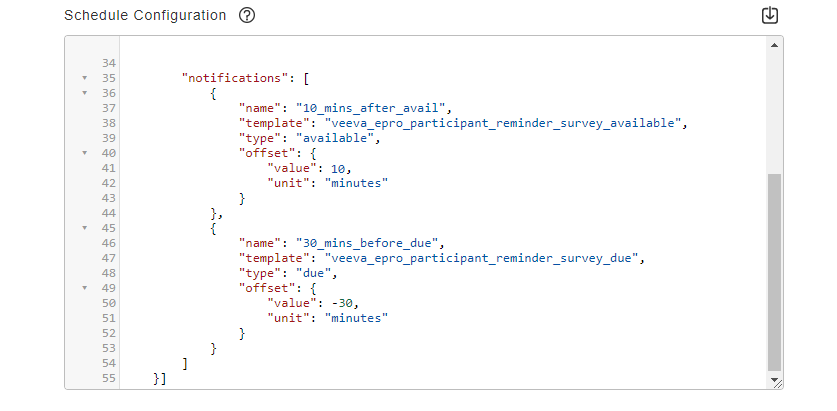
Notification Configuration
This feature allows sponsors to configure notifications to be sent to participants and sites. Notifications can be sent to participants when a survey task is available, is coming due, or has been missed. Notifications can be sent to sites when a participant has yet to complete a survey task or when they miss one or more survey tasks.
See the Configuring Schedules and Notifications page in the Clinical Operations Help for more information.
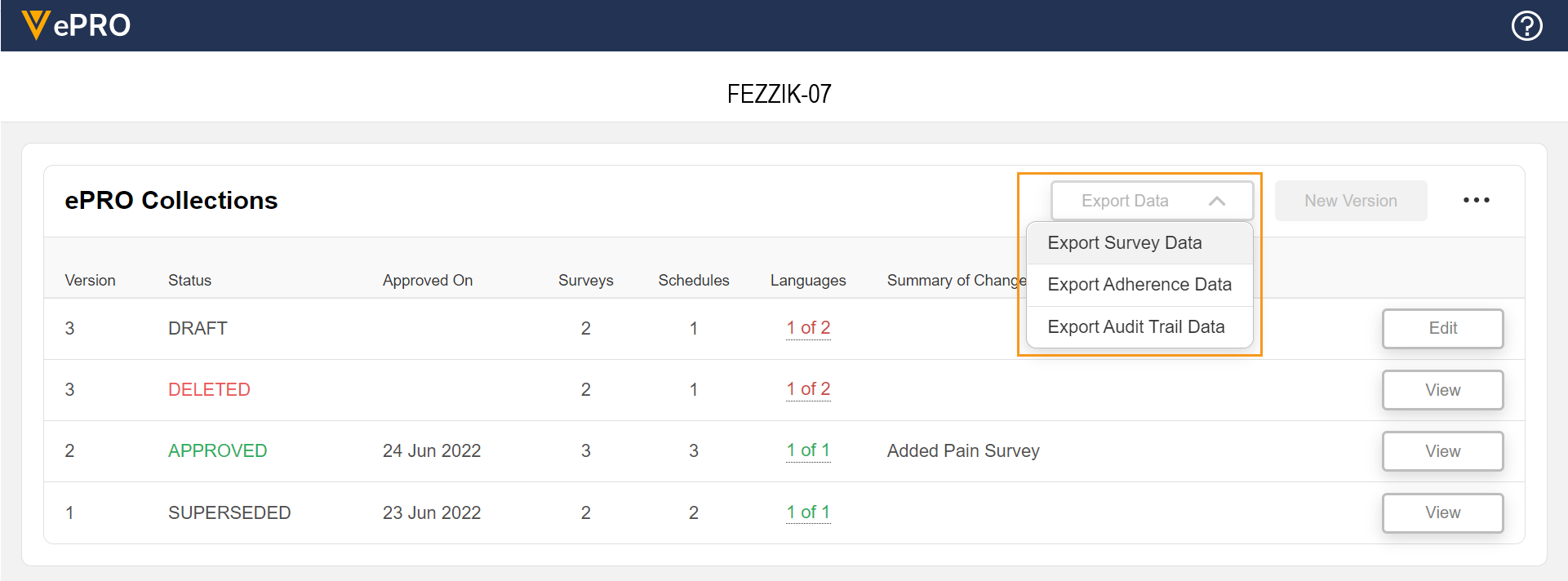
Sponsor Data Export
This feature allows sponsors to export survey data, adherence data, and audit trail data for a study.
See the Exporting Survey Response, Adherence, and Audit Trail Data page in Clinical Operations Help for more information.
Veeva ePRO in SiteVault
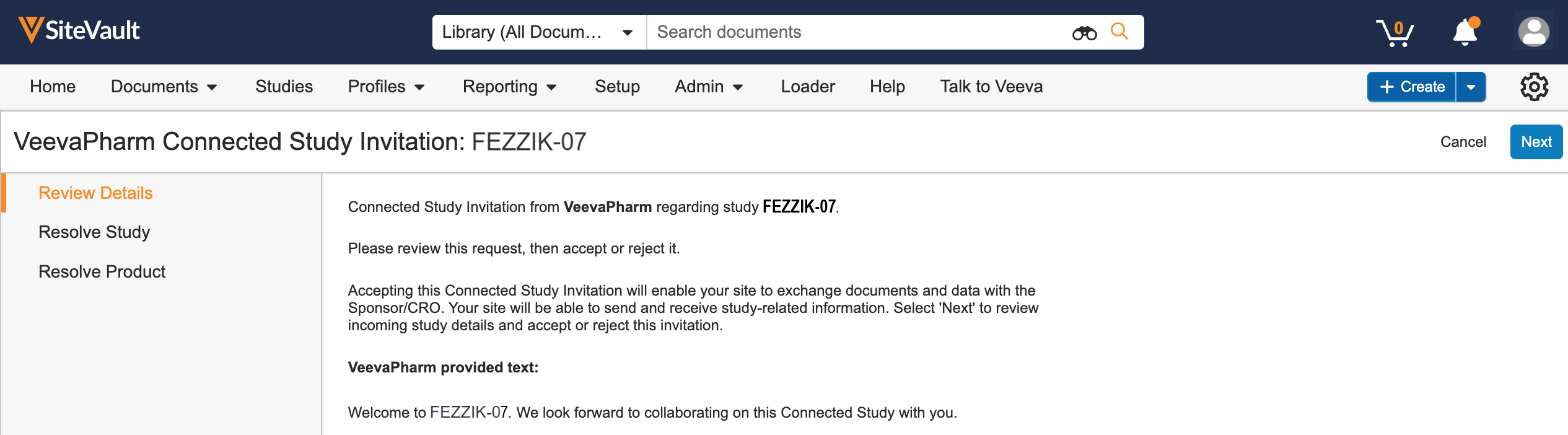
ePRO Connection
This feature allows sites to connect a study in SiteVault to a sponsor’s ePRO by entering a sponsor-provided ePRO code or by accepting a Site Connect agreement.
This feature is only available to sites conducting a study whose sponsor is utilizing Veeva ePRO in Clinical Operations.
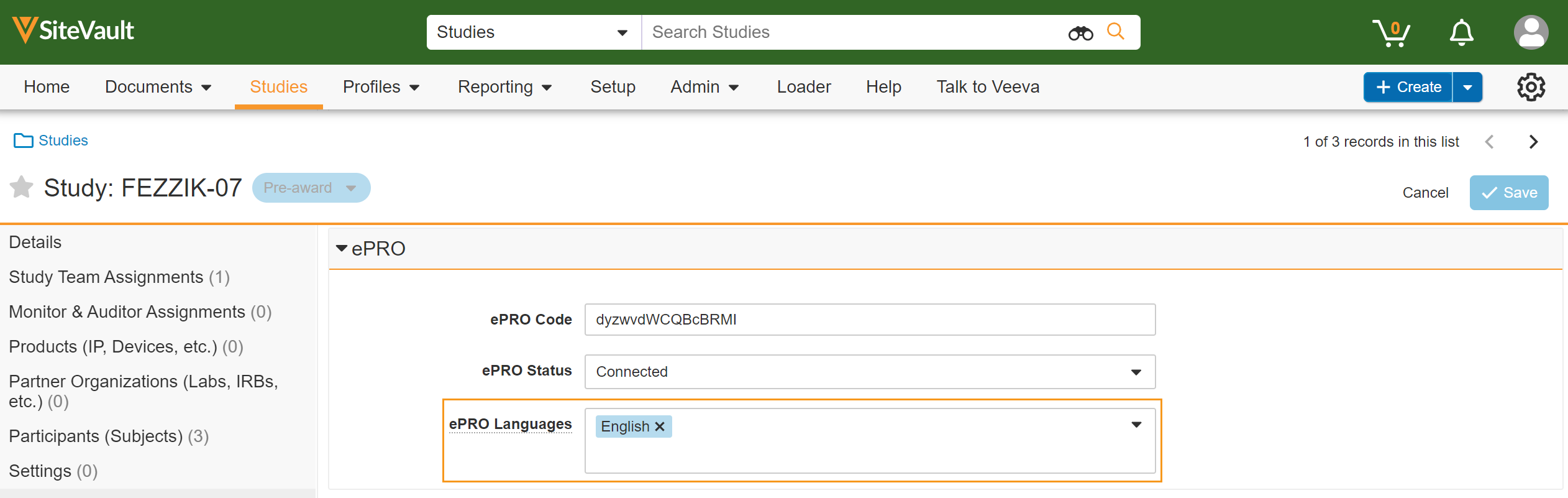
Language Management
This feature allows sites to add and remove supported languages for a study’s ePRO in SiteVault. Users are prevented from adding languages that aren’t supported by the study or from removing languages that have already been approved by their IRB/EC.
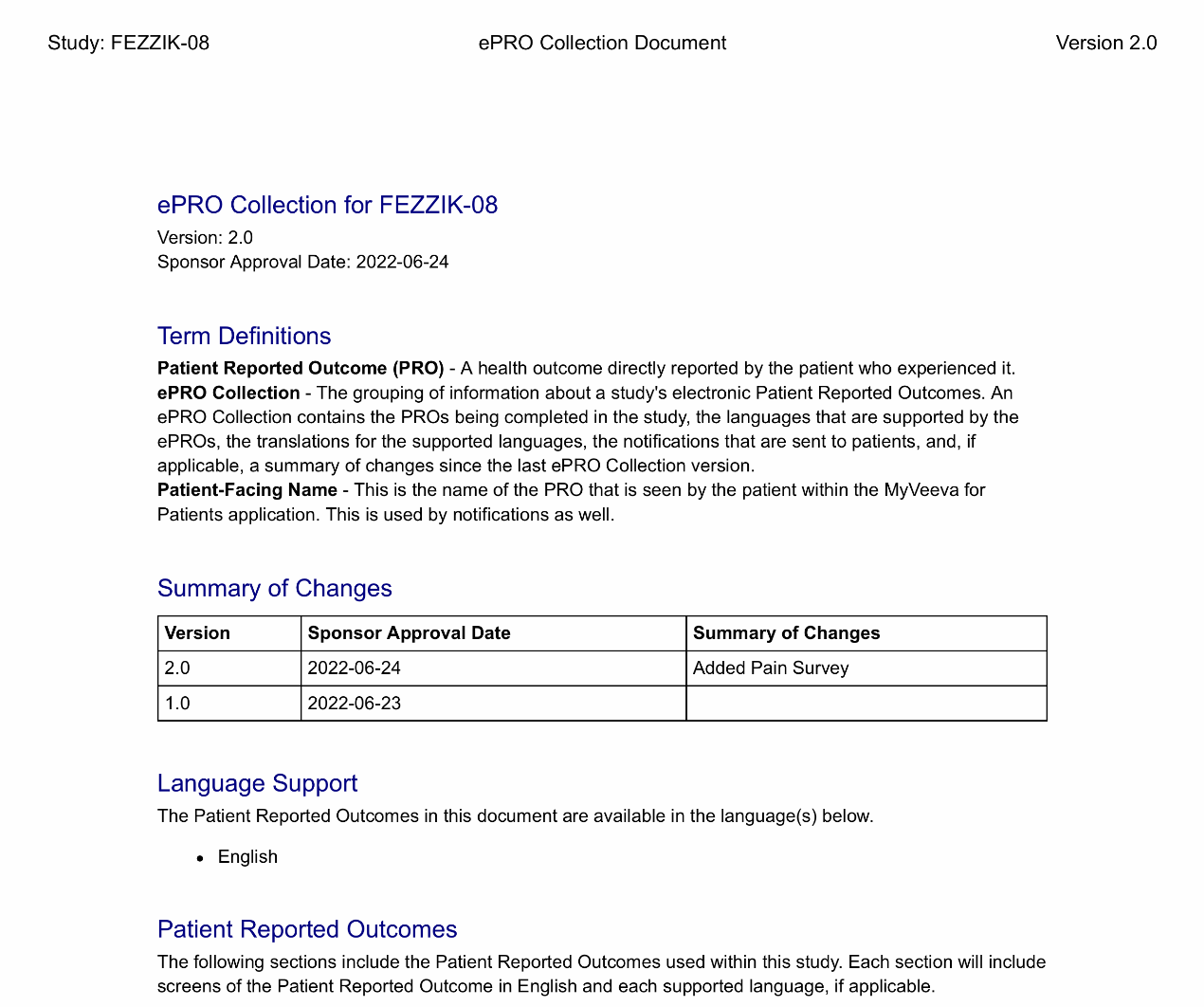
ePRO Collection Document
This feature allows sites to download an ePRO collection document that contains all versions of a study’s ePRO, screenshots of all surveys in each language supported by the site, applicable notifications in each language supported by the site, and more. The ePRO collection document can be used to aid local IRB/EC approval. This document must be approved before participants can be enabled with ePRO.
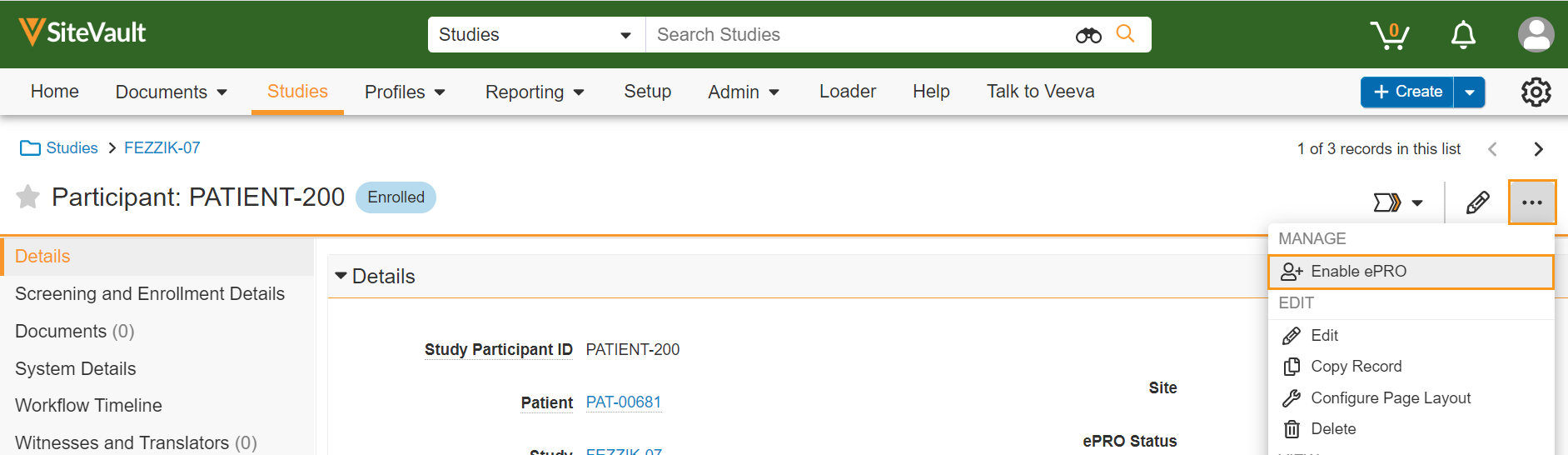
ePRO Enablement
This feature allows sites to enable and disable ePRO for participants when an approved ePRO collection document exists for a study.
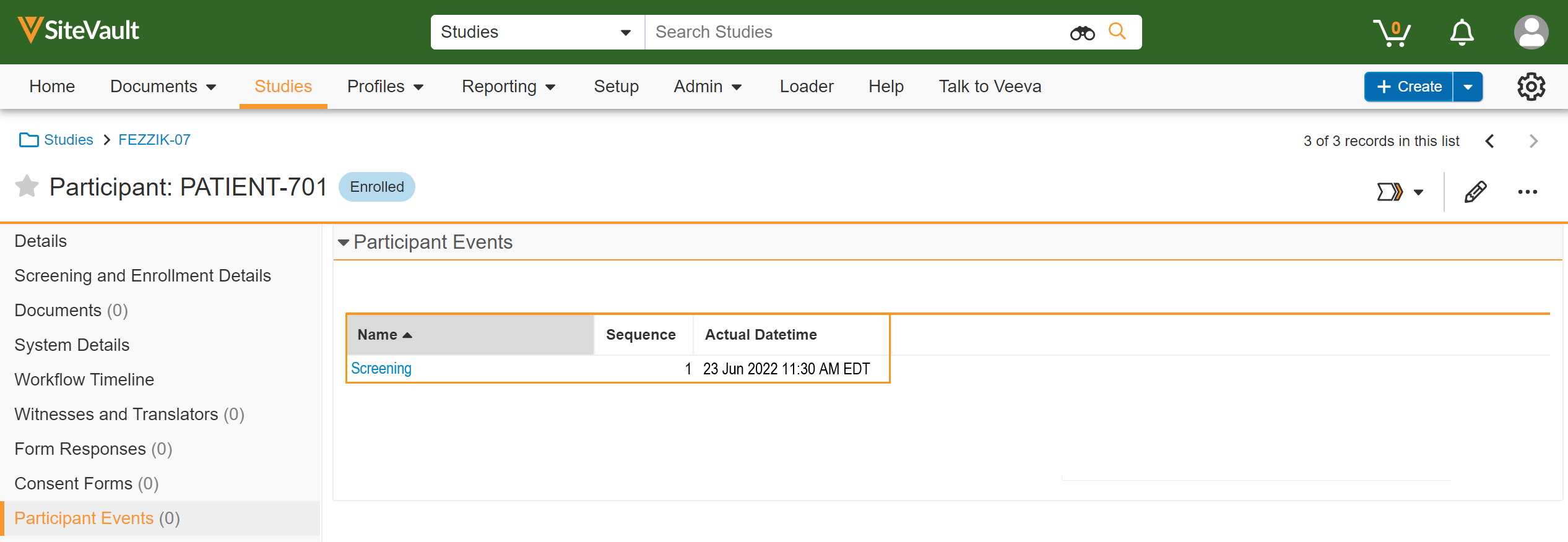
Participant Event Management
This feature allows sites to trigger schedules for a participant by adding, updating, or removing actual date/times for participant events.
Site ePRO Notifications
This feature allows sites to receive ePRO-related notifications. Notifications may be received for successful or unsuccessful attempts to connect ePRO, update languages, approve ePRO collections, and more. Notifications may also be received when a participant has an upcoming survey to complete or has missed one or more surveys, if those notifications are configured for a study’s ePRO.
Site Data Export
This feature allows sites to export survey data, adherence data, and audit trail data for a study.
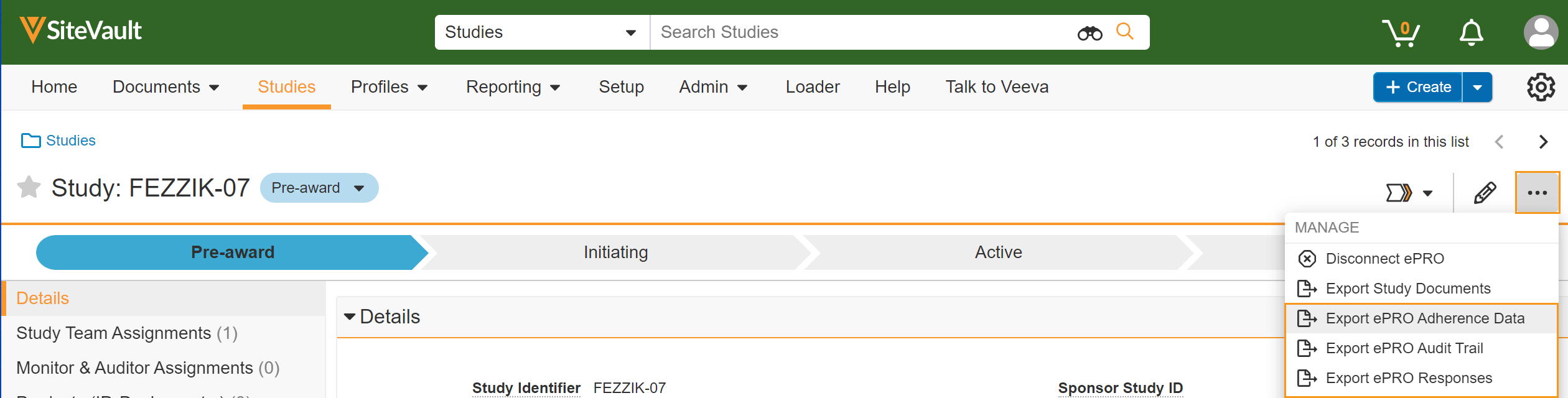
Veeva ePRO in MyVeeva for Patients
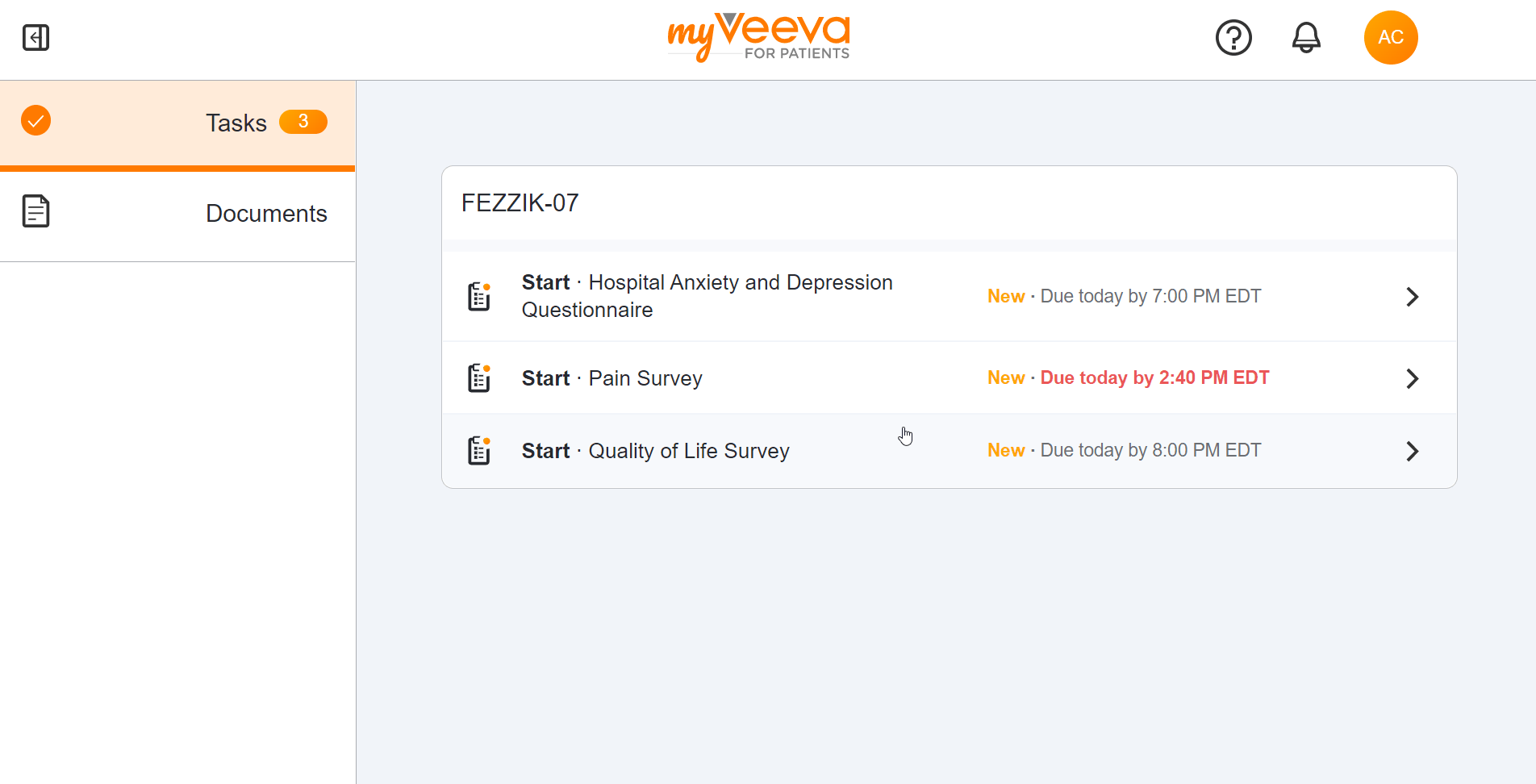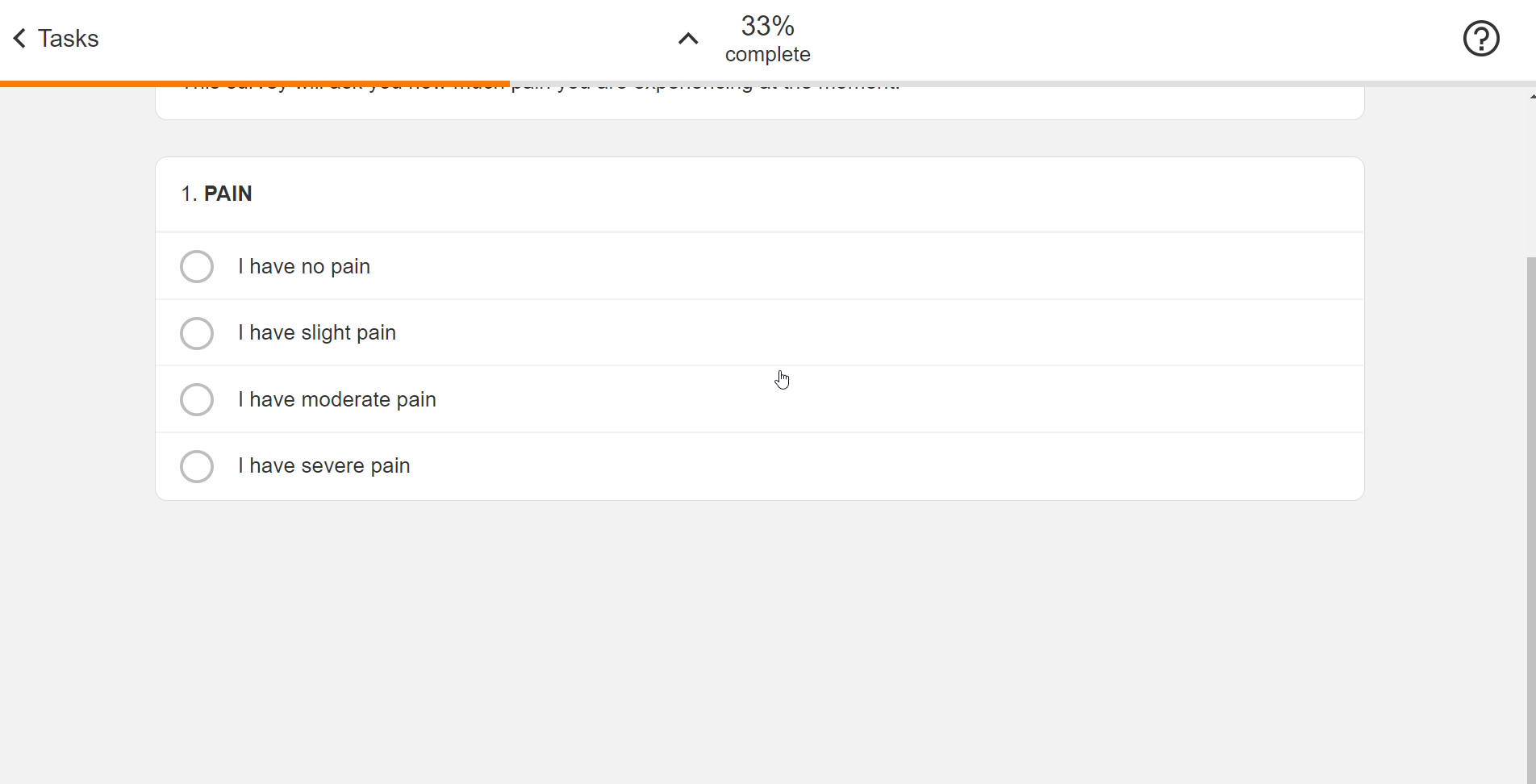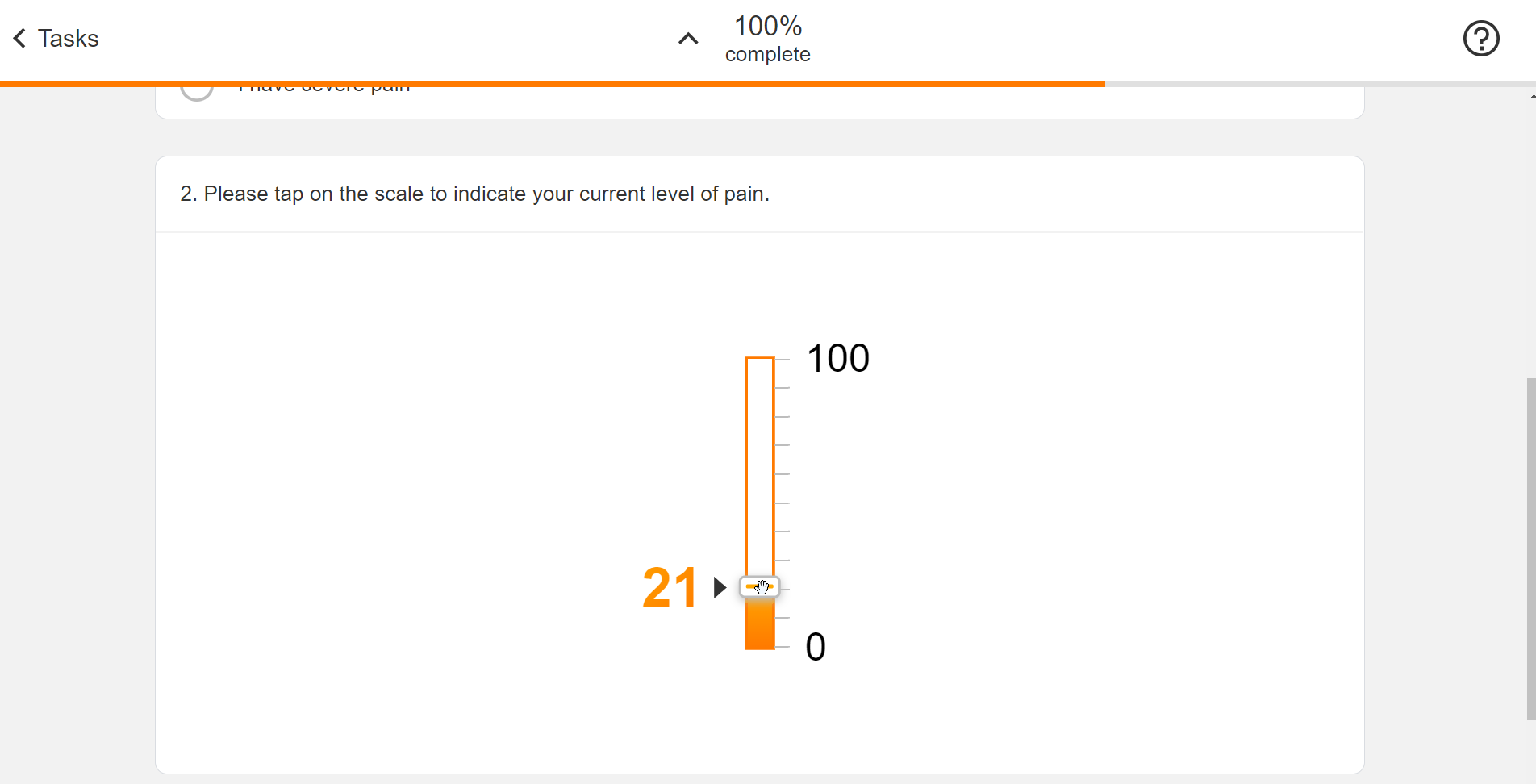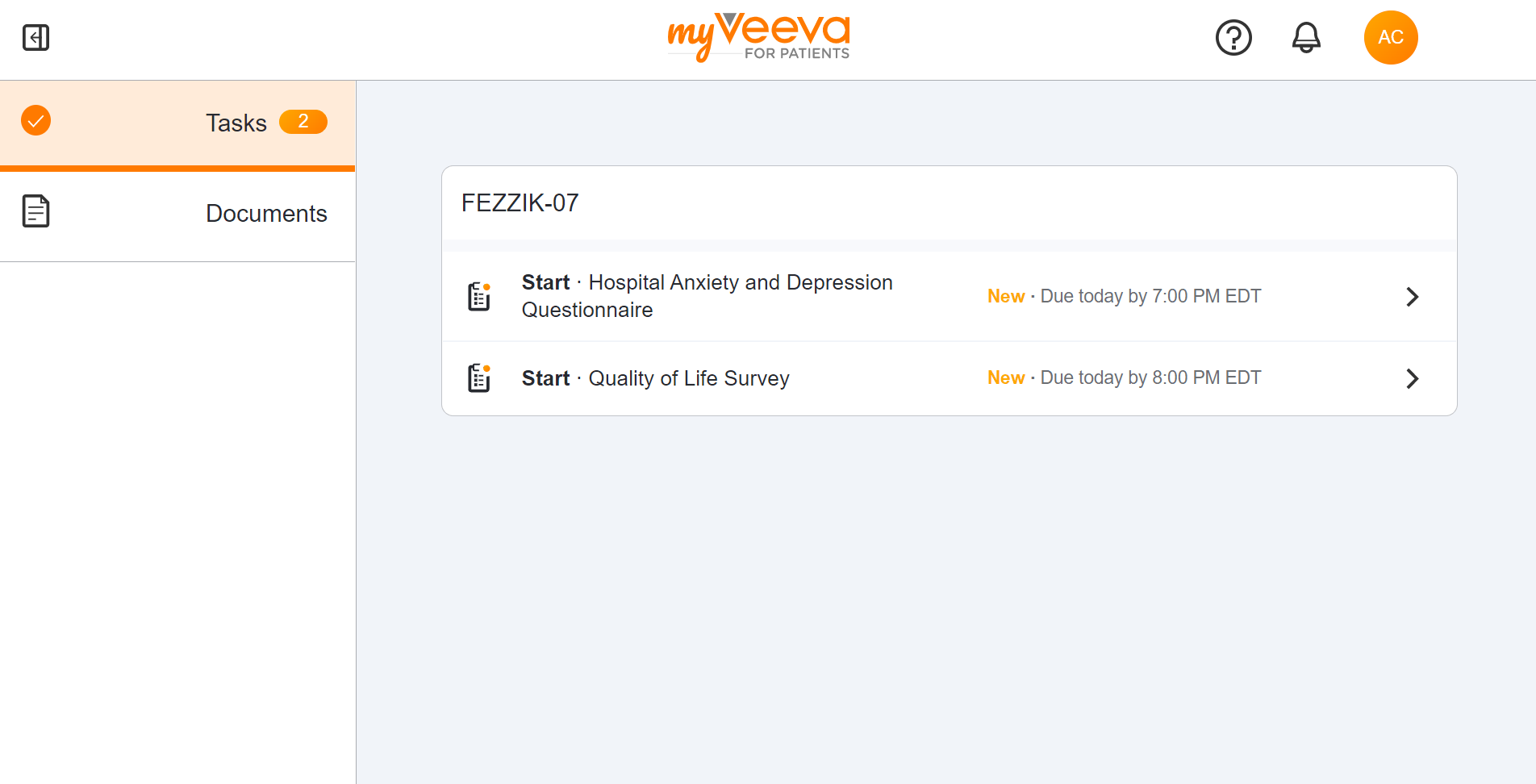
Survey Tasks
This feature allows participants to receive their assigned surveys as tasks in MyVeeva for Patients. Participants can see the name of the survey, the due date/time, and whether or not they’ve already started it by viewing their tasks list. Surveys can be started or resumed when selected from the tasks list.
This feature is only available to participants of a study whose sponsor is utilizing Veeva ePRO in Clinical Operations.
See the Surveys help topic in the MyVeeva for Patients Help for more information.

Survey Notifications
This feature allows participants to receive notifications related to their surveys in MyVeeva for Patients. Participants can receive a welcome notification once they are enabled with ePRO and a study completion notification once they complete a study. Additional notifications can be received if configured by the sponsor for a study, such as when new surveys become available, when a survey’s due date/time is approaching, and when a survey is missed.
See the Surveys help topic in the MyVeeva for Patients Help for more information.
Survey Completion
This feature allows participants to start, resume, and submit their surveys in MyVeeva for Patients. Survey progress is measured with a progress bar that shows a percentage of completion, and participants can automatically skip questions if conditions have been configured by sponsors. Participants return to their tasks list when they submit a survey. If a participant exits a survey before submitting it, their progress will be saved and they can resume at any point before the due date/time.
See the Surveys help topic in the MyVeeva for Patients Help for more information.
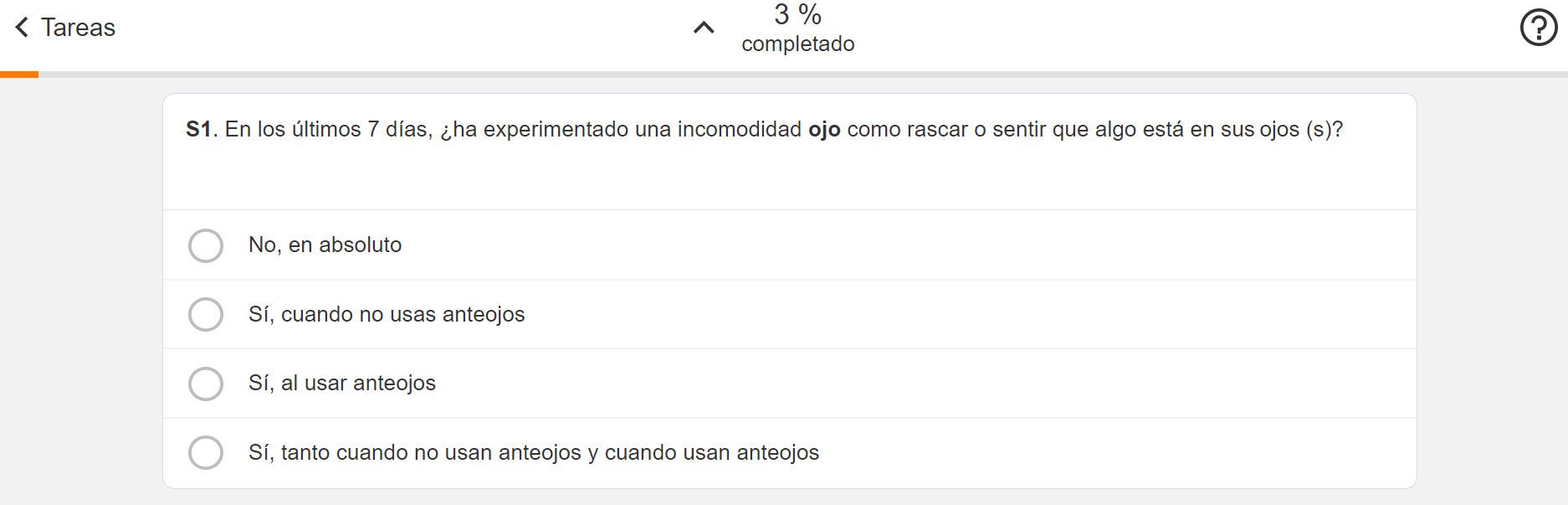
Survey Translations
This feature allows participants to view and complete their assigned surveys in any language that their site supports. Participants can also be sent survey-related notifications in any language that their site supports.
See the Surveys help topic in the MyVeeva for Patients Help for more information.
MyVeeva for Patients Mobile
Mobile Deferred Write for Authenticated Users
This feature allows MyVeeva for Patients mobile app users who lose connection to cellular network or Wi-Fi to resume and submit assigned surveys while their device is offline. Their offline data is saved locally to their device until reconnection to the network is established, at which point their submitted data is sent to the server.
Users cannot resume or submit a survey while offline unless the survey was already in progress at the time of connection loss. Users cannot begin new surveys while offline.
See the Surveys help topic in the MyVeeva for Patients Help for more information.
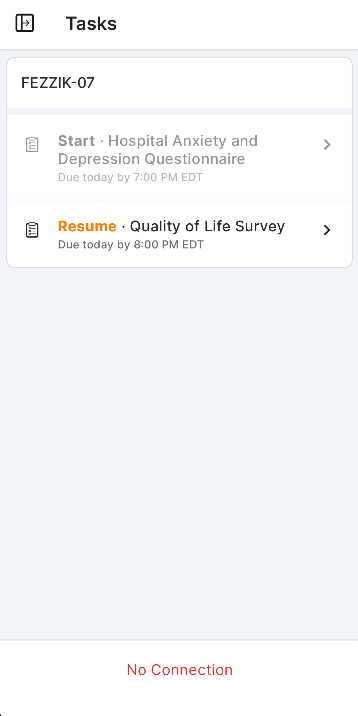
Android App
MyVeeva for Patients is now available as a mobile app on Android devices. Participants and other users can use the app to begin, resume, and submit surveys, view and download non-eConsent documents, and sign, decline, and download eConsent forms in an all-new, native Android mobile experience.
This release includes the features currently available in the MyVeeva for Patients web and iOS applications and the 22R2 features that impact MyVeeva for Patients users.
Participants and their signatories can download the Android app from Google Play.
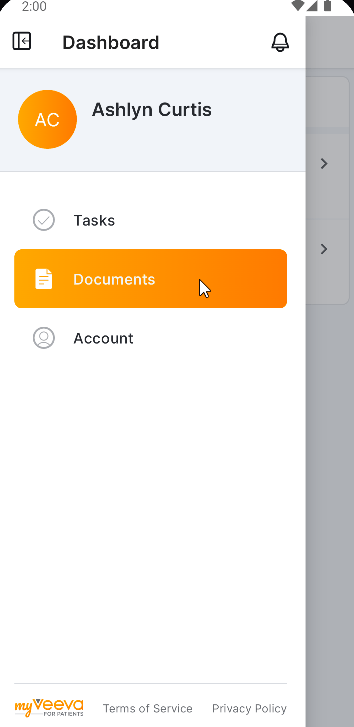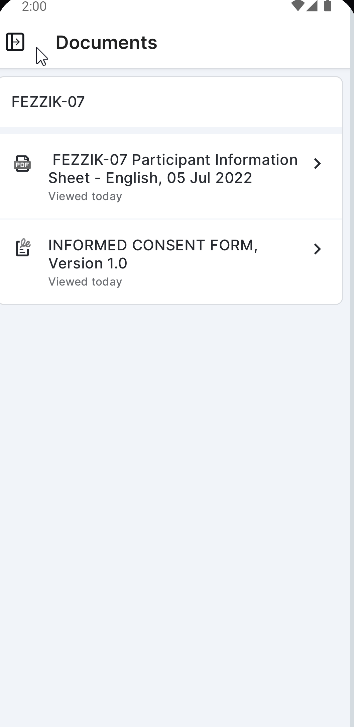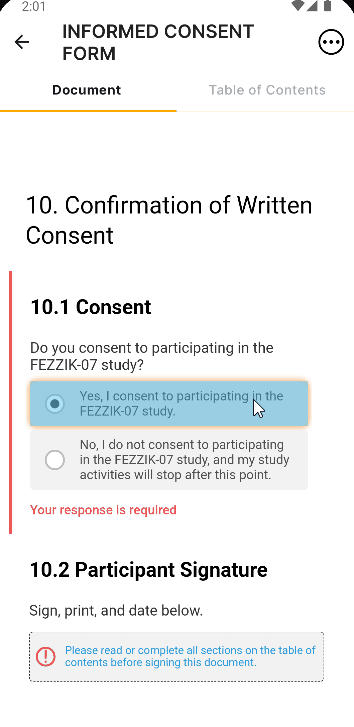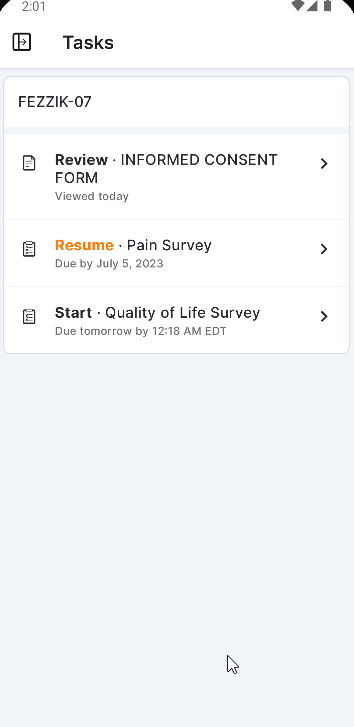
MyVeeva for Patients Platform
User Time Zone Preference
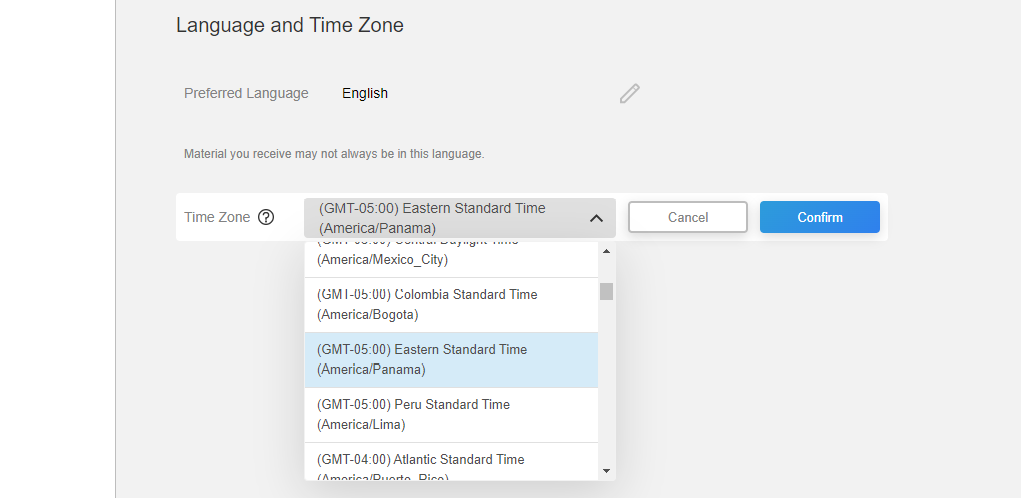
This feature allows a MyVeeva user to set their time zone in their account settings. Notifications and tasks are sent or assigned based on the setting if they are specific to the user’s time zone instead of a fixed time, for example, a daily diary that must be completed at a specific time in the morning in the user’s time zone. Any timestamps that are displayed in the application will be converted to the user’s preferred time zone.
When a user initially registers an account, their time zone setting is the site’s time zone in SiteVault.
See the Account Settings topic in MyVeeva for Patients Help for more information.
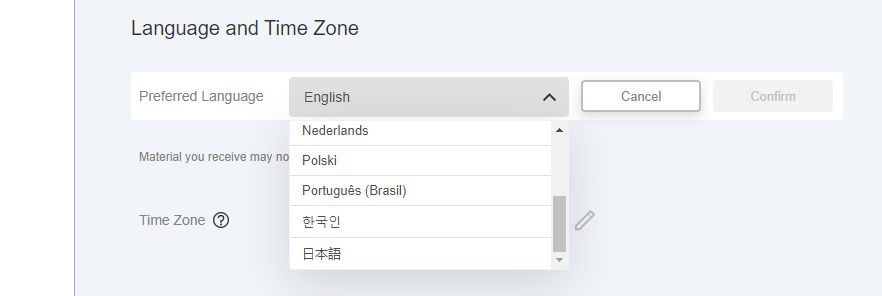
22R2.0 Translations and New Languages (Dutch, Japanese, Korean, and Portuguese)
This feature allows MyVeeva for Patients users to view application text, emails, text messages, and the terms of use and privacy policy in Dutch, Japanese, Korean, and Portuguese (Brazil).
Users can set their preferred language in their account settings. If the language is already set, this functionality is automatically on for the user.
See the Account Settings help topic in MyVeeva for Patients Help for more information.
Additional Enhancements
The following enhancements are now available for MyVeeva for Patients, Veeva eConsent, and Veeva ePRO:
| Component | Users | Description | Number |
|---|---|---|---|
| eConsent - Editor | Site Staff, Sponsor Staff | Multiple-choice question blocks on eConsent forms now require responses by default. Previously, responses to multiple-choice question blocks were optional by default. Single-choice question blocks remain optional by default. | MYVC-4804 |
| eConsent - SiteVault Integration | Site Staff, Sponsor Staff | This feature automatically syncs the ID of a study participant with eConsent response records in Clinical Operations in the event that a study participant's ID is updated by site staff in SiteVault. A participant's response records in Clinical Operations show their new ID each time it's changed by site staff. This feature supports studies that apply sponsor-defined IDs to participants when they transition from candidacy to active participation in the study. If eConsent form responses are submitted before or during this transition, they're synced with the new ID to maintain the consistency of the participant's record throughout the study. | MYVC-8469 |