About the Release
Important Dates
| Date | Event | Description |
|---|---|---|
| March 21 | Pre-Release | The release is deployed to the MyVeeva for Patients pre-release environment and available for customers to explore from pre-release vaults. |
| April 22 | General Release | The release is deployed and generally available. The MyVeeva for Patients validation binder will be available to customers in VeevaDocs at this time. |
Release Information and Impacts
| Resource | Description | Publication History |
|---|---|---|
| MyVeeva for Patients 22R1 Release Impact Assessment |
The release impact assessment analyzes the regulatory, implementation, and user impacts of the MyVeeva for Patients and Veeva eConsent features in this release. |
Updated March 1 |
| What's New |
The What's New information provides detailed explanations of the new features for MyVeeva for Patients and Veeva eConsent. |
Published March 4 |
| Fixed Issues |
The Fixed Issues list documents issues that affected previous versions or the pre-release and are fixed in this release. |
Available April 8 |
| Known Issues |
The MyVeeva for Patients Known Issues page lists issues that affect this release or previous ones and aren't fixed yet. |
Available April 8 |
| Maintenance Releases |
The MyVeeva for Patients Maintenance Releases page lists releases that fix issues affecting the general release. |
Available with the first maintenance release |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new features across the Digital Trials Platform:
|
See links |
What’s New
Veeva eConsent
Form Response Data Shared With Sponsor
This feature allows sponsors to view consent and response data for studies across sites in a structured format. When a MyVeeva user submits a signature or response on a consent form connected to a sponsor, MyVeeva for Patients sends the de-identified participant response data to the Clinical Operations vault. Sponsors can then report on and aggregate the data as needed.
The eConsent form must be shared with an import code or Site Connect by the sponsor with the site. Only signatures and responses from content blocks on the sponsor form are shared with the sponsor. Blocks added by the site are not shared. If a site wants to use a sponsor form as a template for another purpose, site staff can disconnect the form from the sponsor.
See the Consent Form Settings section in Clinical Operations Help for more information.

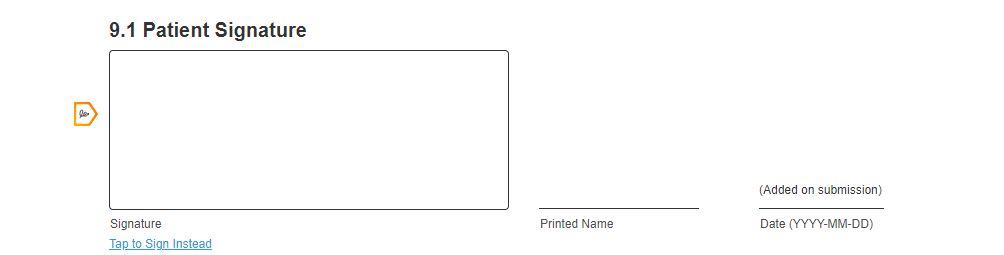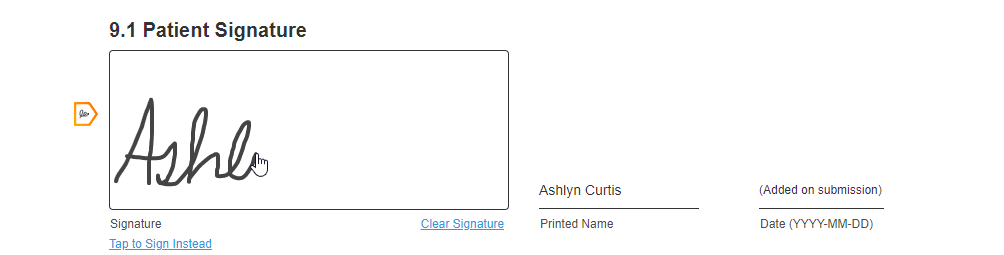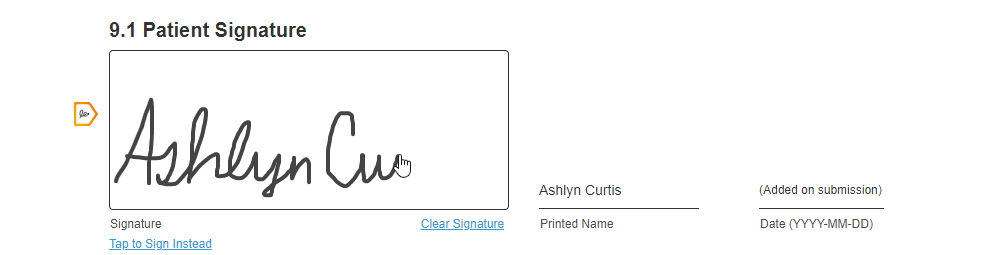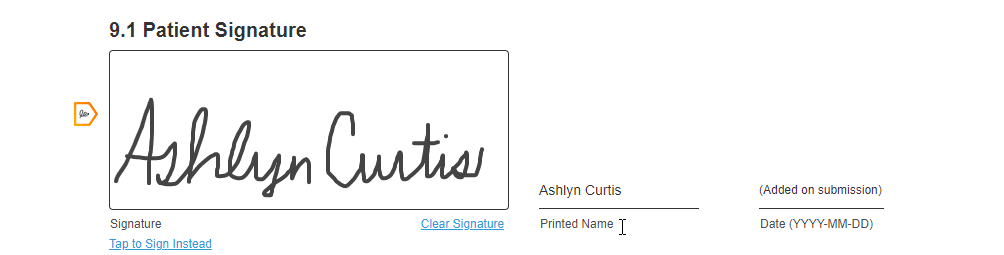
Hand-Drawn Signature
This feature allows sites and sponsors to require a hand-drawn signature for a consent form. This feature also allows registered MyVeeva users to hand-draw their signature on any consent form, which some users prefer to feel a sense of ownership over their consent.
If site or sponsor staff want to require a hand-drawn signature for a consent form, they can edit the form to toggle the Requires Hand-Drawn Signature setting to on in the Veeva eConsent editor.
See the following help topics for more information:
- Consent Forms in the MyVeeva for Patients Help
- Consent Form Settings section in Clinical Operations Help
Simple Signing
This feature allows participants and other signatories to sign and submit consent forms in person without registering a user account. Being able to sign without an account enables signatories in demographics without email addresses or mobile phones to use Veeva eConsent within compliance regulations, increasing the scope of studies and participants that Veeva eConsent supports.
In SiteVault, site staff can now send eConsent forms without contact information. When a MyVeeva user is unregistered, the system requires the MyVeeva user to sign during an in-person consenting session and to draw their signature in place of dual-factor authentication. This feature is only available in the web app, which supports the in-person consent flow.
See the following help topics for more information:
- Consent Forms in the MyVeeva for Patients Help
- Consenting a Participant In Person in the SiteVault Help

Add Site Countersignature to MyVeeva .PDF Files
This feature allows MyVeeva users to view and download countersigned .PDF files of consent forms. When site staff countersign a consent form in SiteVault, the system updates the .PDF file in MyVeeva for Patients to include the site signature.
This feature applies only to consent forms that are countersigned after the 22R1.0 release. Consent forms countersigned before the release will continue to include only the MyVeeva user signatures.
See the following help topics for more information:
- Documents in the MyVeeva for Patients Help
- Countersigning eConsent Forms in the SiteVault Help
MyVeeva for Patients Platform
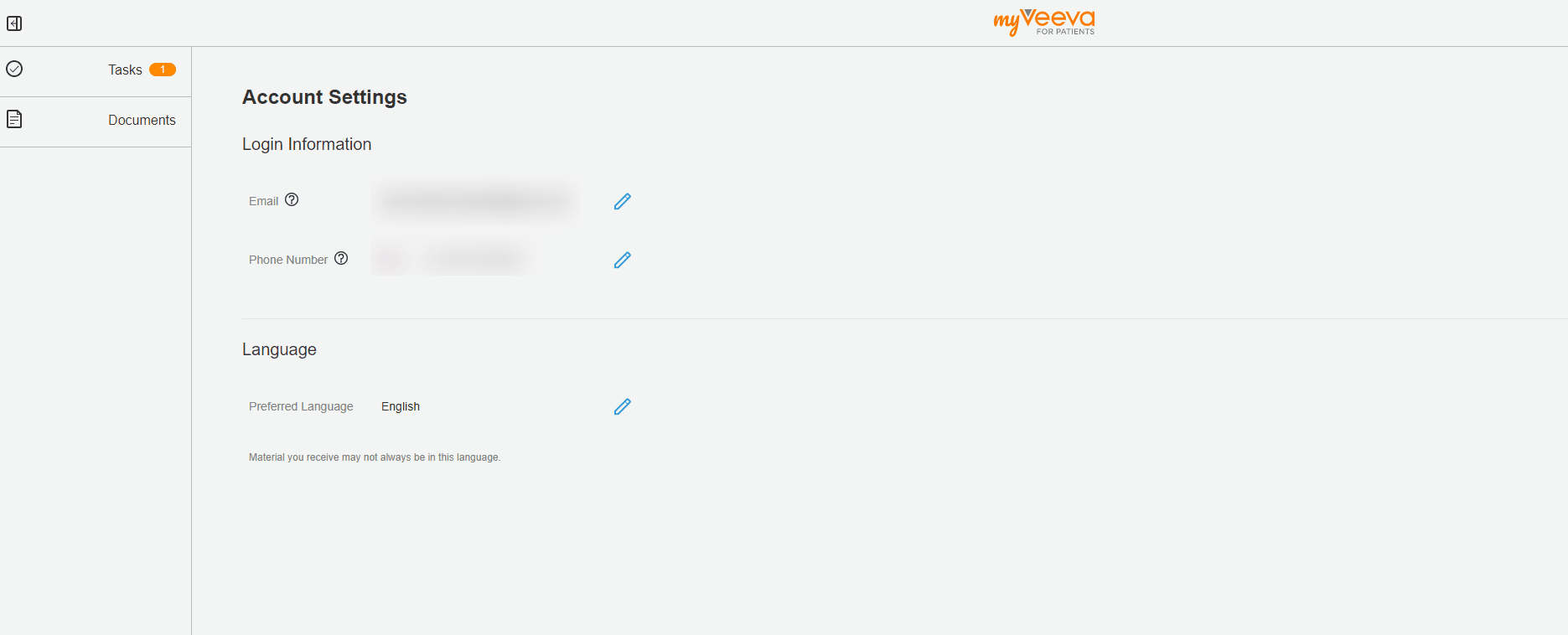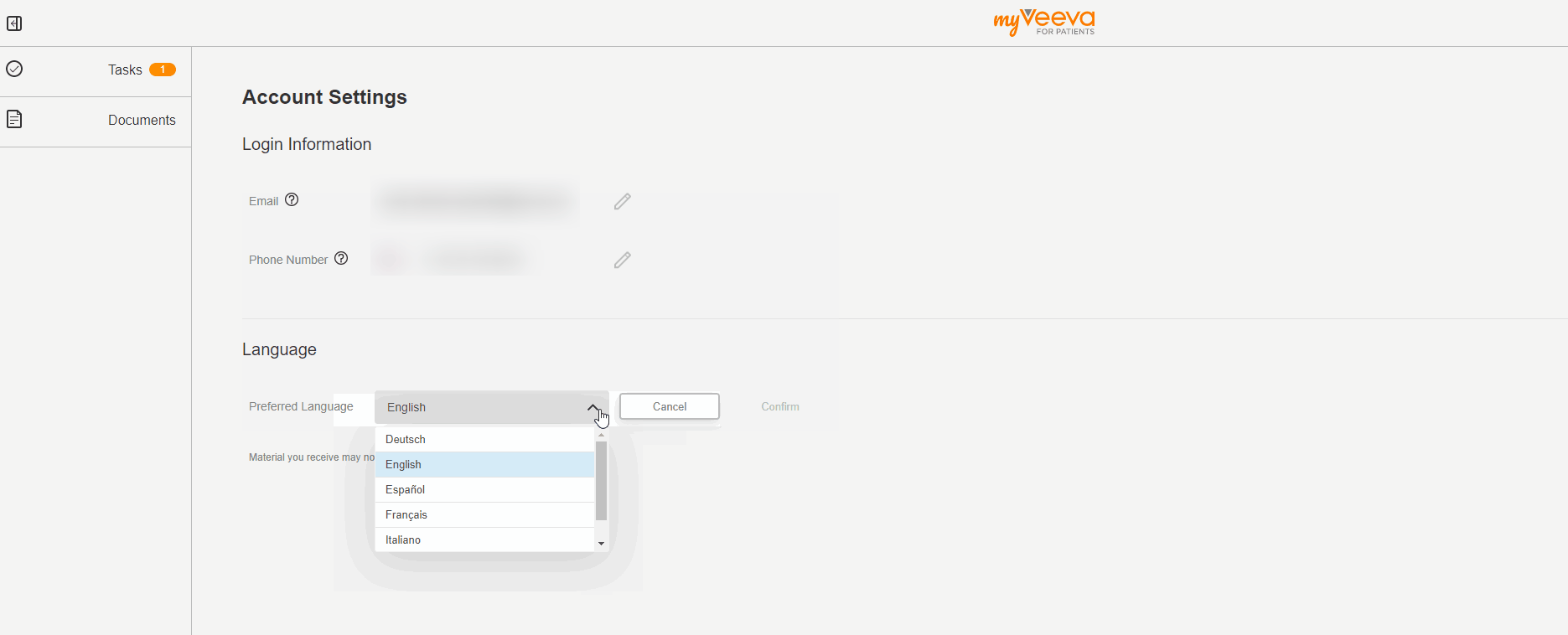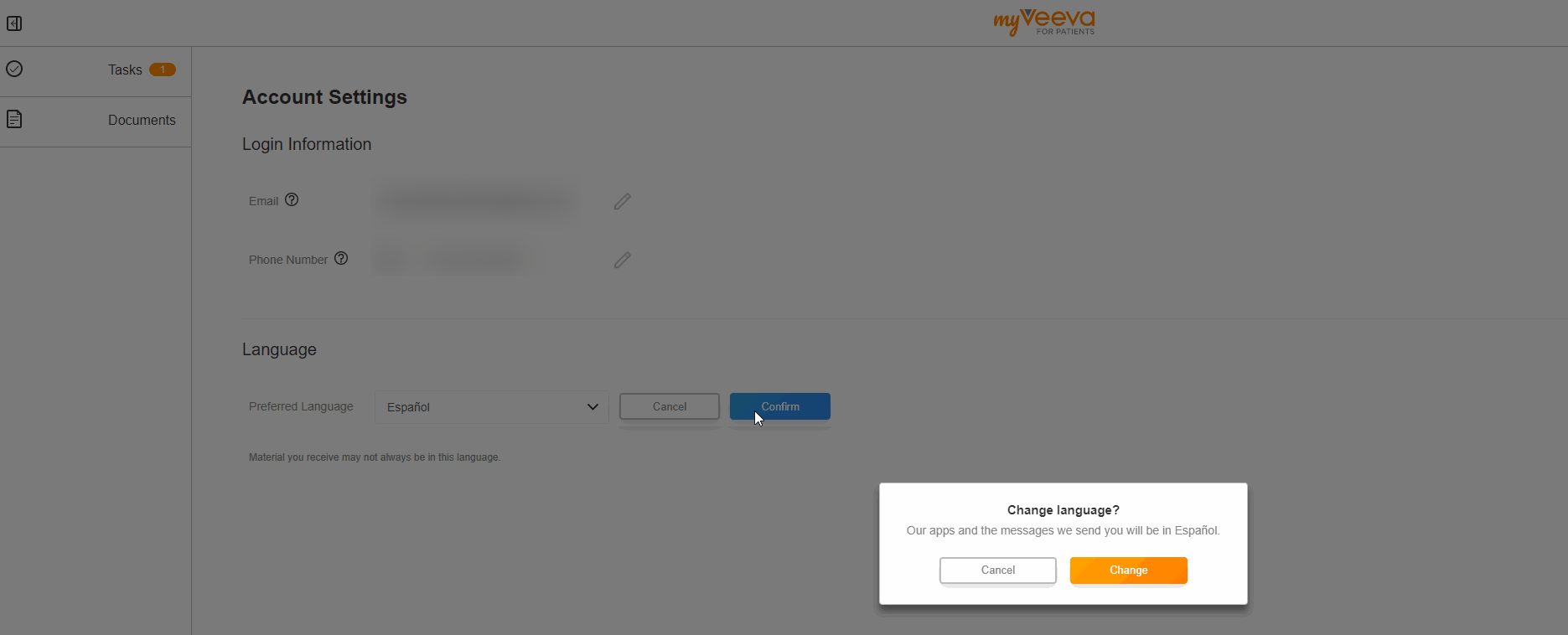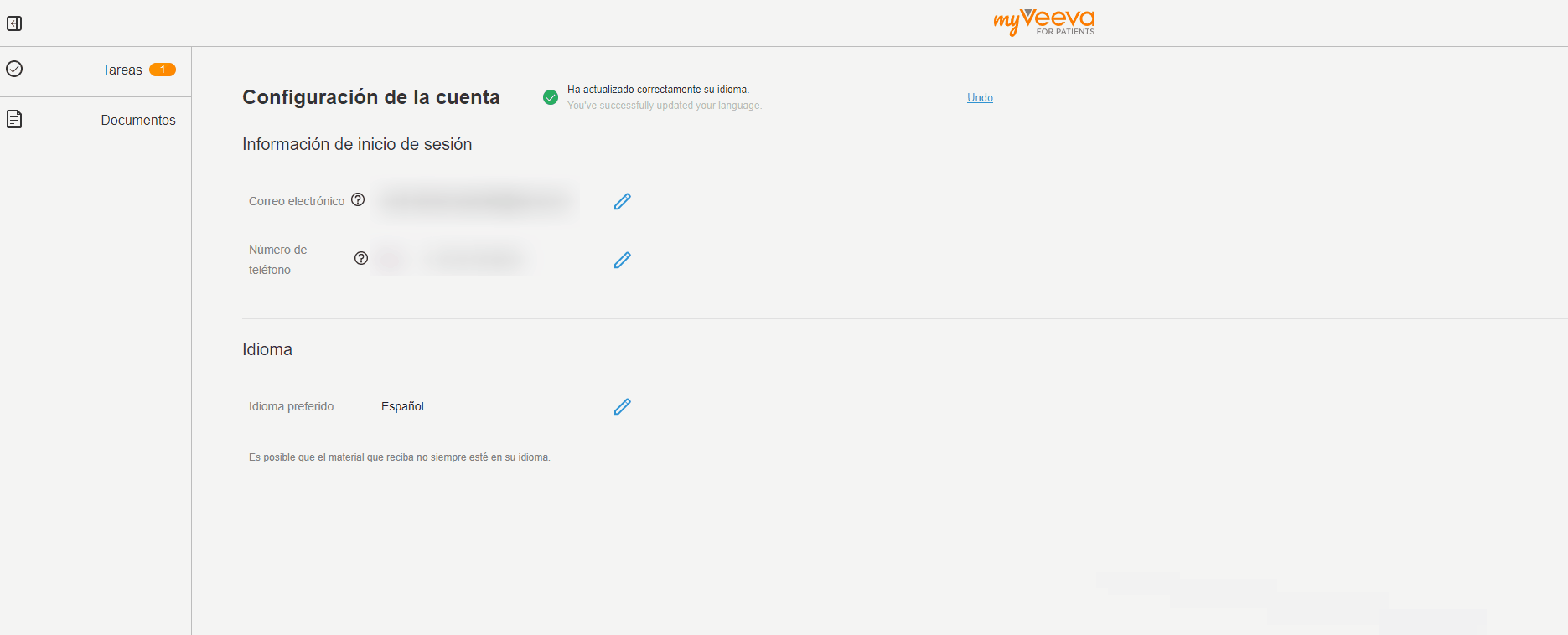
User Language Preference
This feature allows MyVeeva users to update their preferred language in their account settings. When they change their preferred language, the user interfaces of both the web and mobile apps are displayed in the new language. Notifications such as emails and text messages are also sent in the new language.
The MyVeeva user can select any supported language. Currently, English, French, German, Italian, Polish, and Spanish are supported.
See the Account Settings topic in MyVeeva for Patients Help for more information.
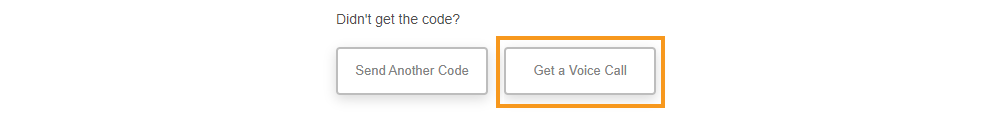
Voice Option for Two-Factor Authentication
This feature allows MyVeeva users to receive two-factor authentication codes in voice calls as a backup for text messages. Sometimes, MyVeeva users can’t receive codes via text message, often because of delays in their wireless carrier’s SMS delivery system. When users can’t receive text messages, they can’t log in, submit signed consent forms, or confirm account setting changes. Wireless carriers prioritize voice calls over SMS messages, so voice calls are more likely to be received when text messages aren’t.
See the Account Settings and Consent Forms help topics in MyVeeva for Patients Help for more information.
Additional Enhancements
The following enhancements are now available for MyVeeva for Patients and Veeva eConsent:
| Component | Users | Description | Number |
|---|---|---|---|
| eConsent - Editor | MyVeeva Users, Site Staff, Sponsor Staff | This feature allows sponsor and site staff to import Microsoft Word™ documents that include font colors and highlights into the eConsent editor. | MYVC-4326 |
| Platform - Audit Trail | Veeva | With this feature, the system records the previous and new values when a MyVeeva user updates their email address or phone number in their account settings. The system encrypts the values as personally identifiable information (PII). | MYVC-6037 |
| Platform - User Profile | MyVeeva Users | This feature includes user interface enhancements for error messages for user account settings in the MyVeeva for Patients web app. | MYVC-6036 |