Release Considerations Veeva eConsent is planned to be generally available in SiteVault and available to early adopters in Clinical Operations in 21R2. If you're interested in becoming an early adopter, contact Veeva support to learn more. Future release dates and information are subject to change.
About the Release
The following dates apply to this release:
- August 6: The 21R2 release of MyVeeva for Patients and the Veeva eConsent editor is deployed and available. The MyVeeva for Patients 21R2 validation binder will be available in VeevaDocs to SiteVault Enterprise and Clinical Operations customers at this time.
- August 16: After the SiteVault change request and validation process, the Veeva eConsent functionality is generally available to all sites who use SiteVault. See the SiteVault Release Notes and Validation Documents for more information.
See the following resources for information about the impact of this release on participants, sites, and sponsors:
| Resource | Description | Updated |
|---|---|---|
| MyVeeva for Patients 21R2 Release Impact Assessment |
The release impact assessment analyzes the regulatory, implementation, and user impacts of the MyVeeva for Patients and Veeva eConsent editor features in this release. |
July 13 |
| What's New |
The What's New in 21R2 information below provides detailed explanations of the new features for MyVeeva for Patients and the Veeva eConsent editor. |
July 13 |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new eConsent functionality across the Digital Trials Platform:
|
See links |
What’s New in 21R2
Additional Responses SiteVault Reporting
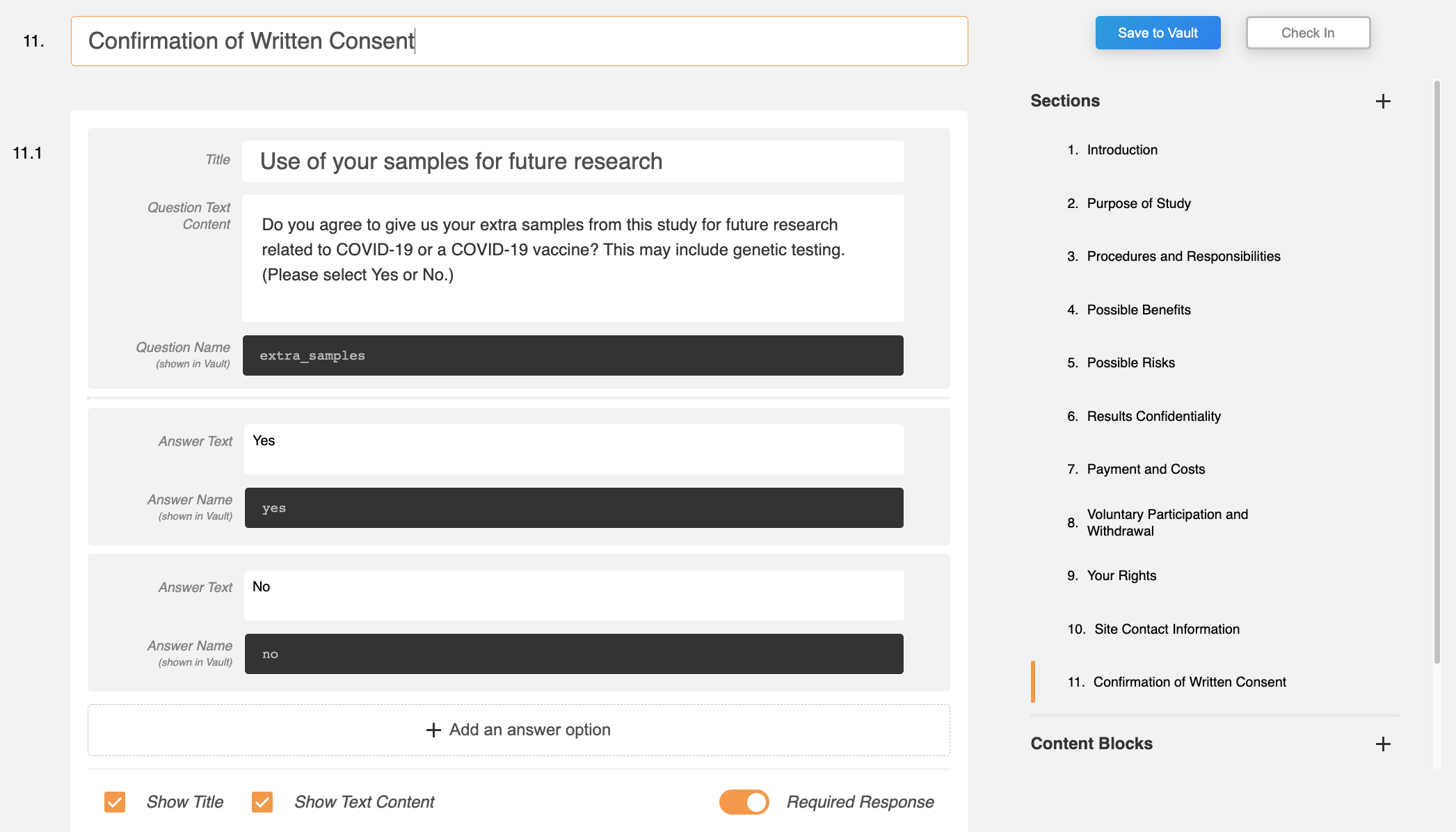
This feature allows site staff to specify names for questions, answers, and signatures in the Veeva eConsent editor and to view and report on participants’ responses in SiteVault.
Embedded Images
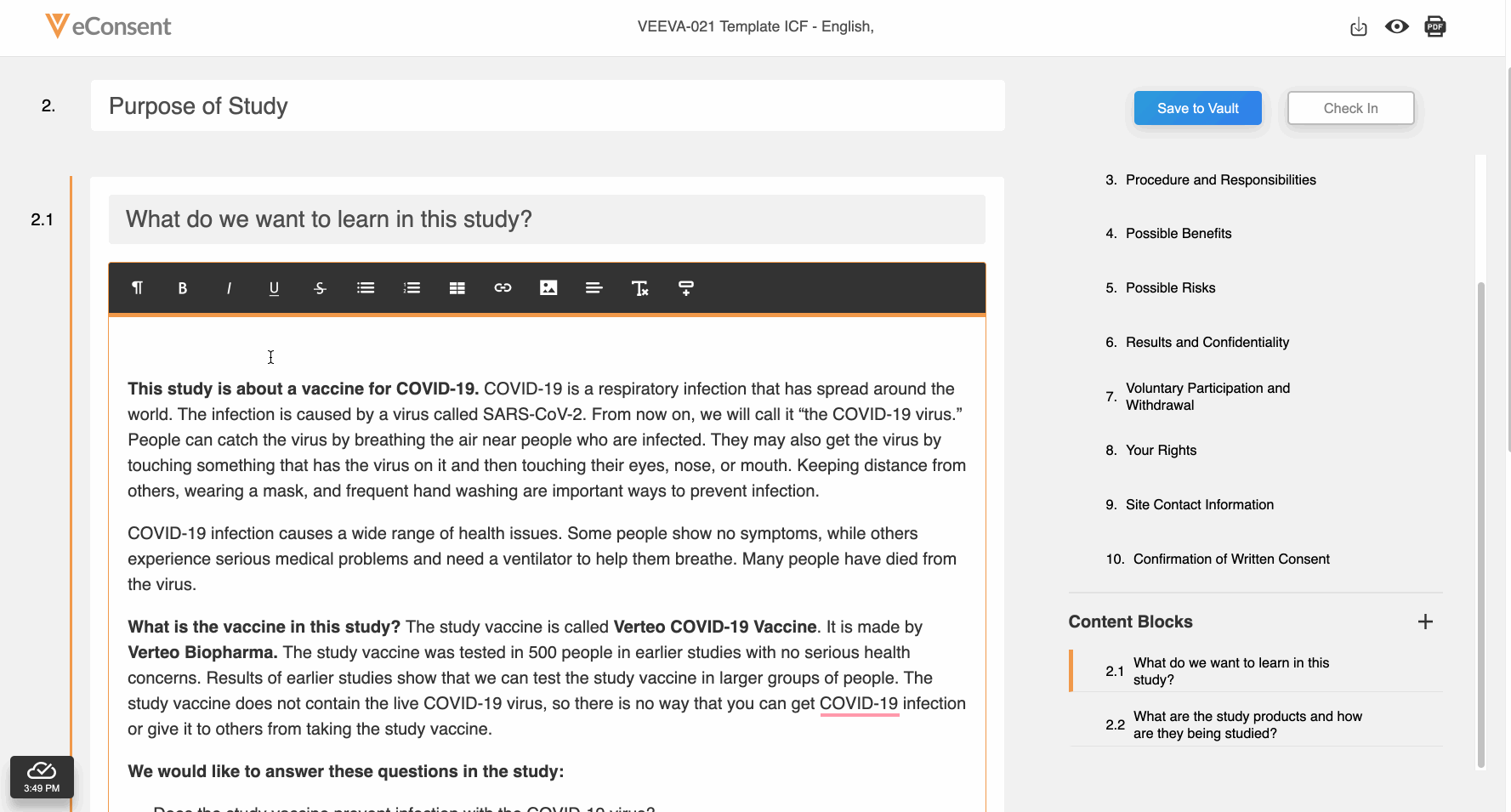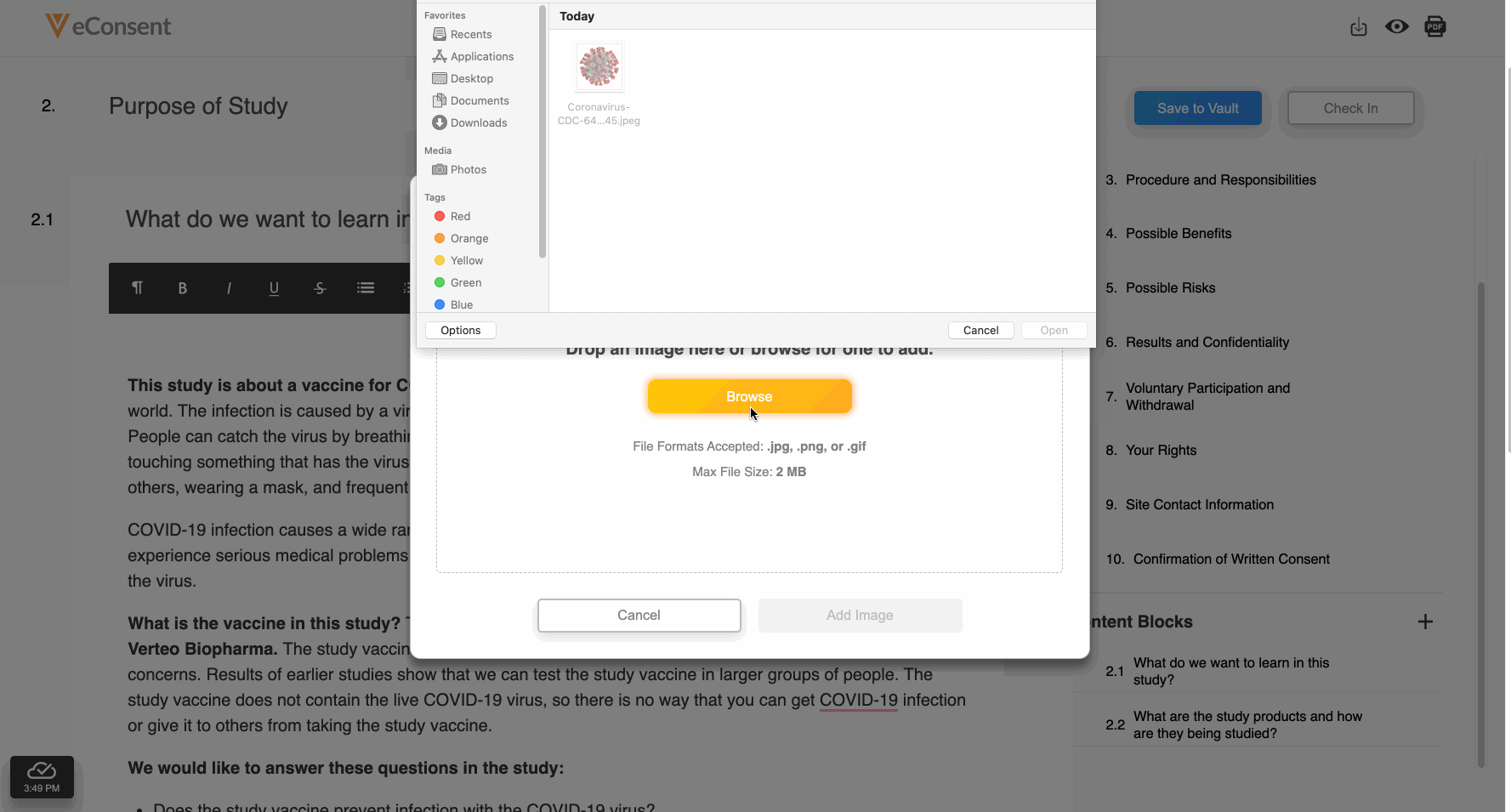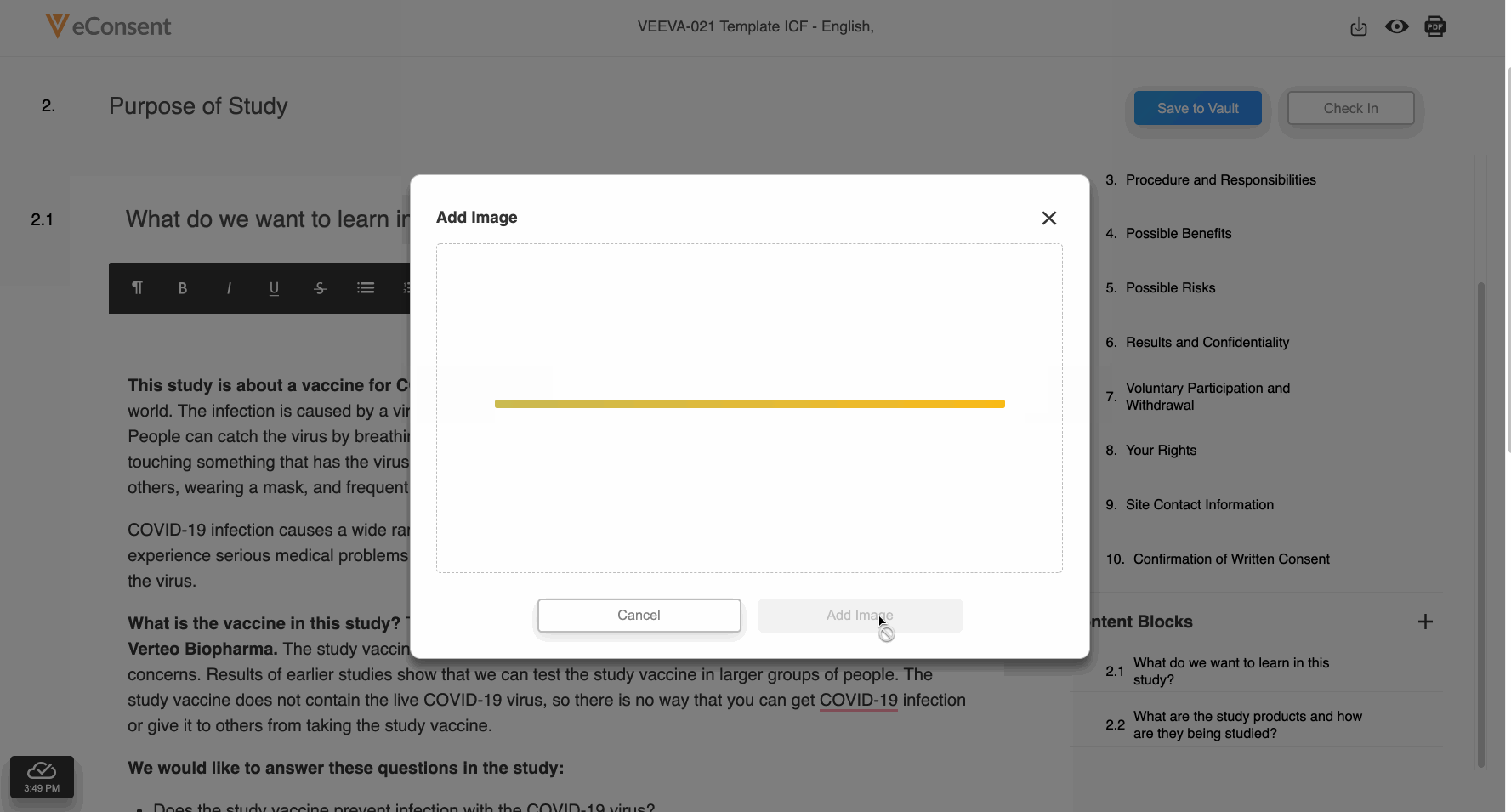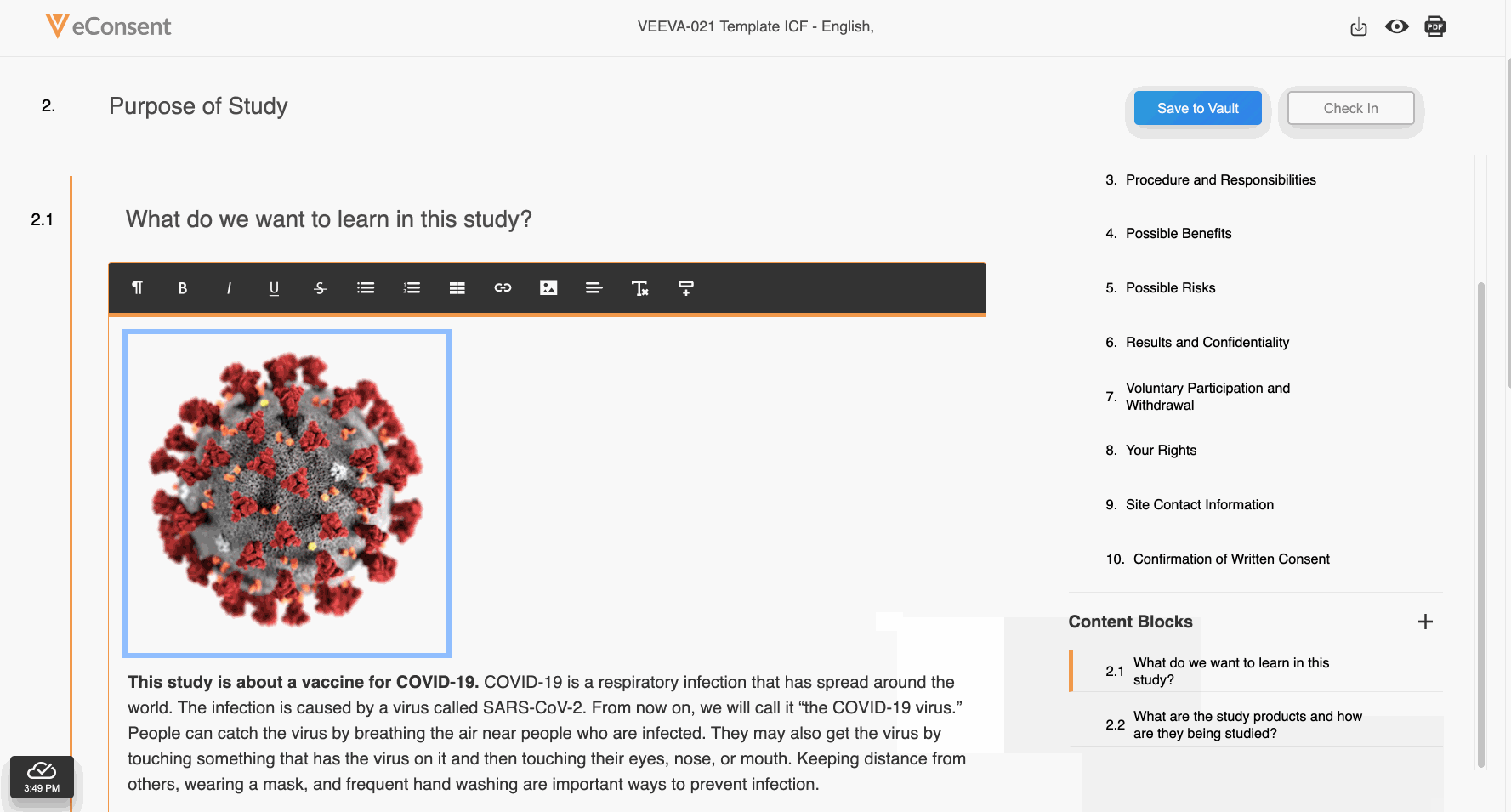
This feature allows site and sponsor staff to add images to Veeva eConsent forms in the Veeva eConsent editor and participants to view them in MyVeeva for Patients. This allows sites and sponsors to upload device diagrams, visit schedules, and other helpful visuals to enhance participants’ experiences during the consenting process. Staff can also add descriptions of images that function as alternative text for screen readers and accessibility.
See the Working With Images section in Clinical Operations Help for more information.
Embedded Videos

Release Considerations The videos feature is currently available only by request. If you want to include a video on an eConsent form, contact Veeva support or your Veeva representative.
This feature allows site and sponsor staff to add videos to Veeva eConsent forms in the Veeva eConsent editor and participants to view them in MyVeeva for Patients. This enables sites and sponsors to embed video content that further explains either study-specific or general medical topics to the participant, for example, the phases of a clinical trial, a summary of informed consent, or an overview of what an MRI visit entails.
Import With Code (Simple Connect)

This feature allows sites and sponsors to transfer Veeva eConsent forms between vaults without Site Connect easily. Sponsors can share forms with sites, and sites can import them and make any site-specific modifications as necessary. Sites can share forms with sponsors in the same way, and the forms can be imported using import codes or the eForm renditions of the forms.
See the Importing eConsent Forms section in Clinical Operations Help for more information.
In-Person Consent

This feature allows site staff to consent participants in person using a site’s device. Site staff can view an in-person consent code from SiteVault, and the participant can scan the QR code or go to the address onscreen to access MyVeeva for Patients. When the participant completes all their documents, they’re prompted to log out or are logged out automatically after 30 seconds or if the browser is closed.
This in-person consenting functionality bypasses the invitation email and makes it easier to consent participants at the office or another location where they may not have their own device.
Participant Preview
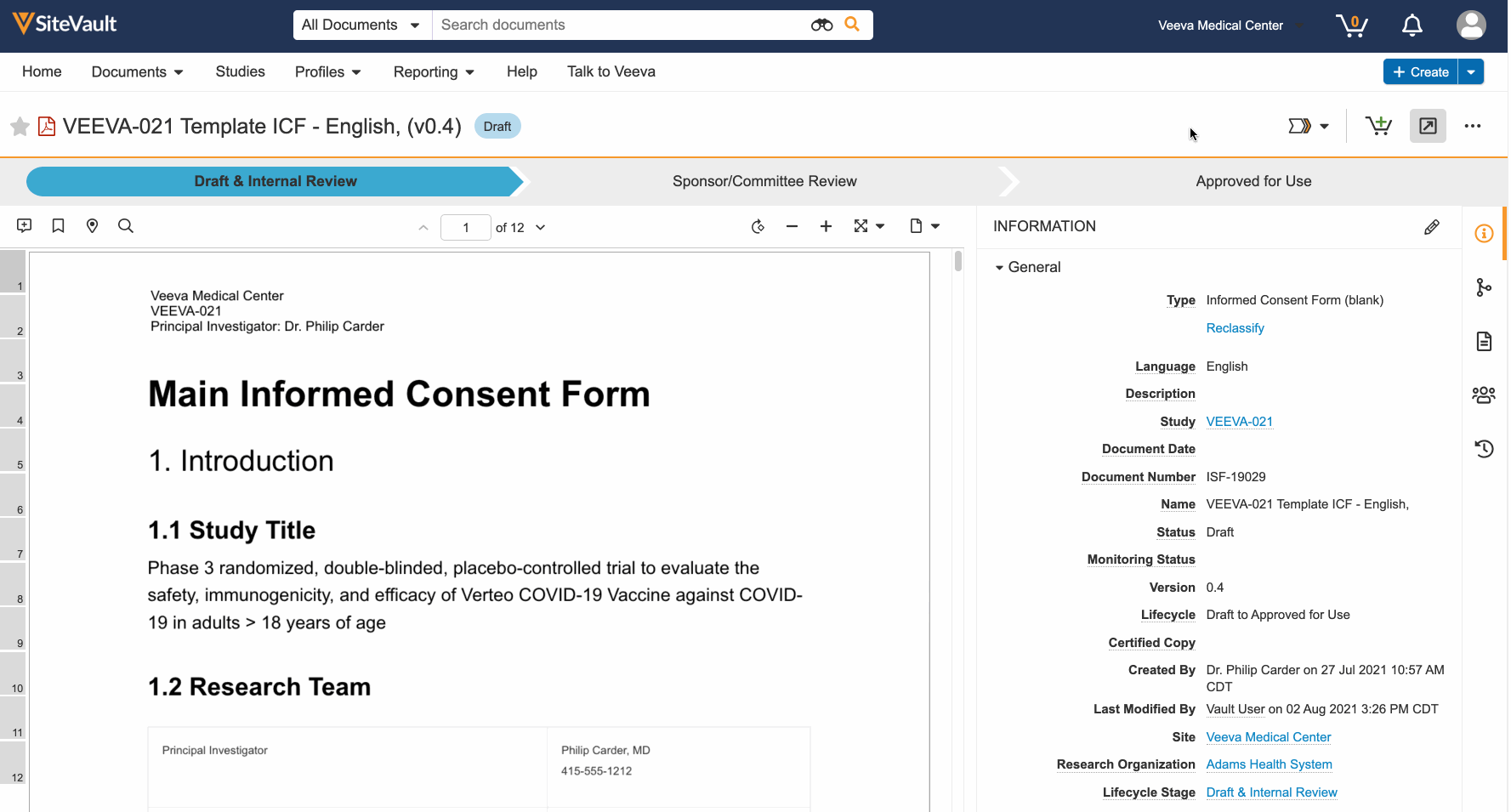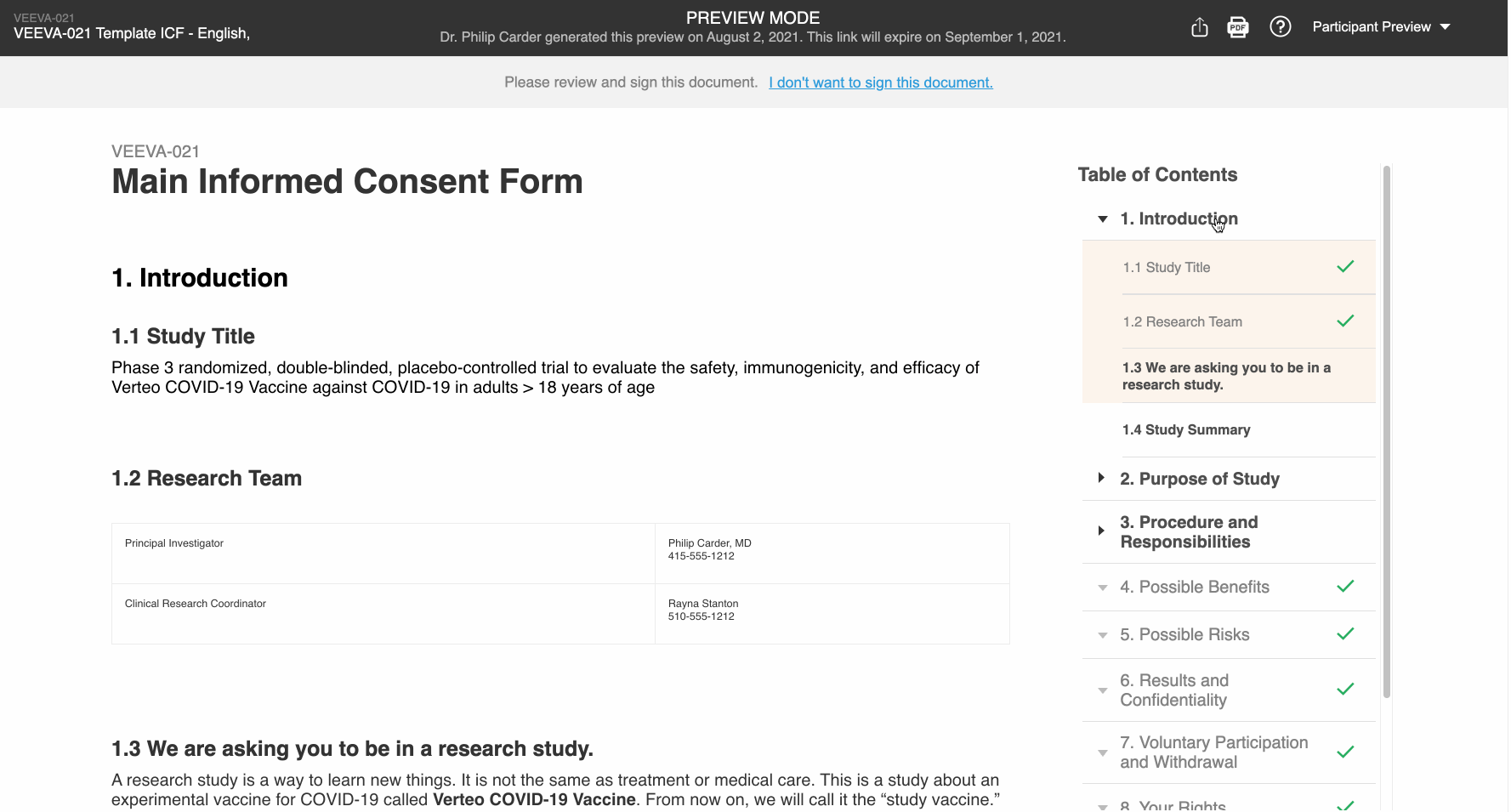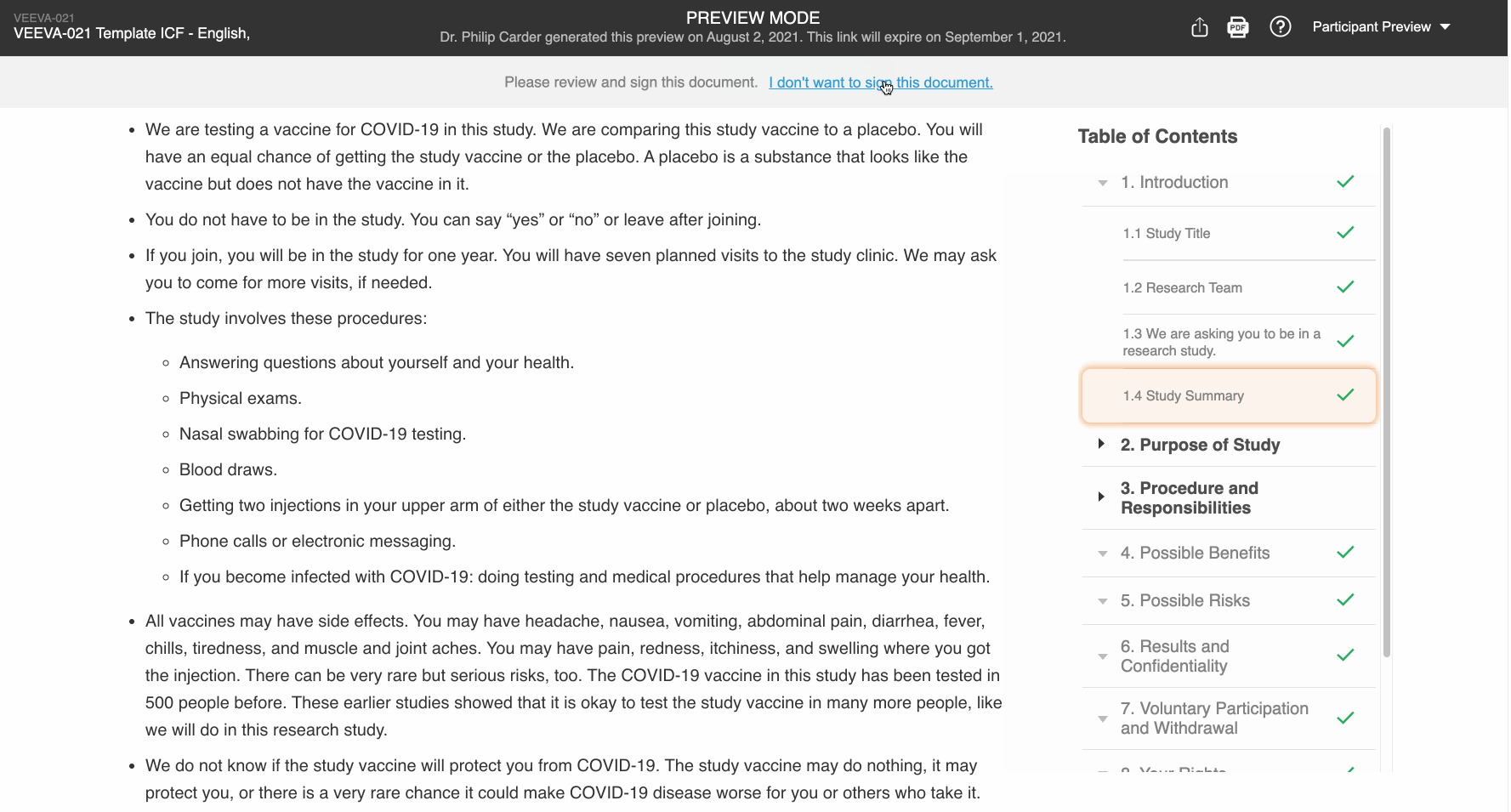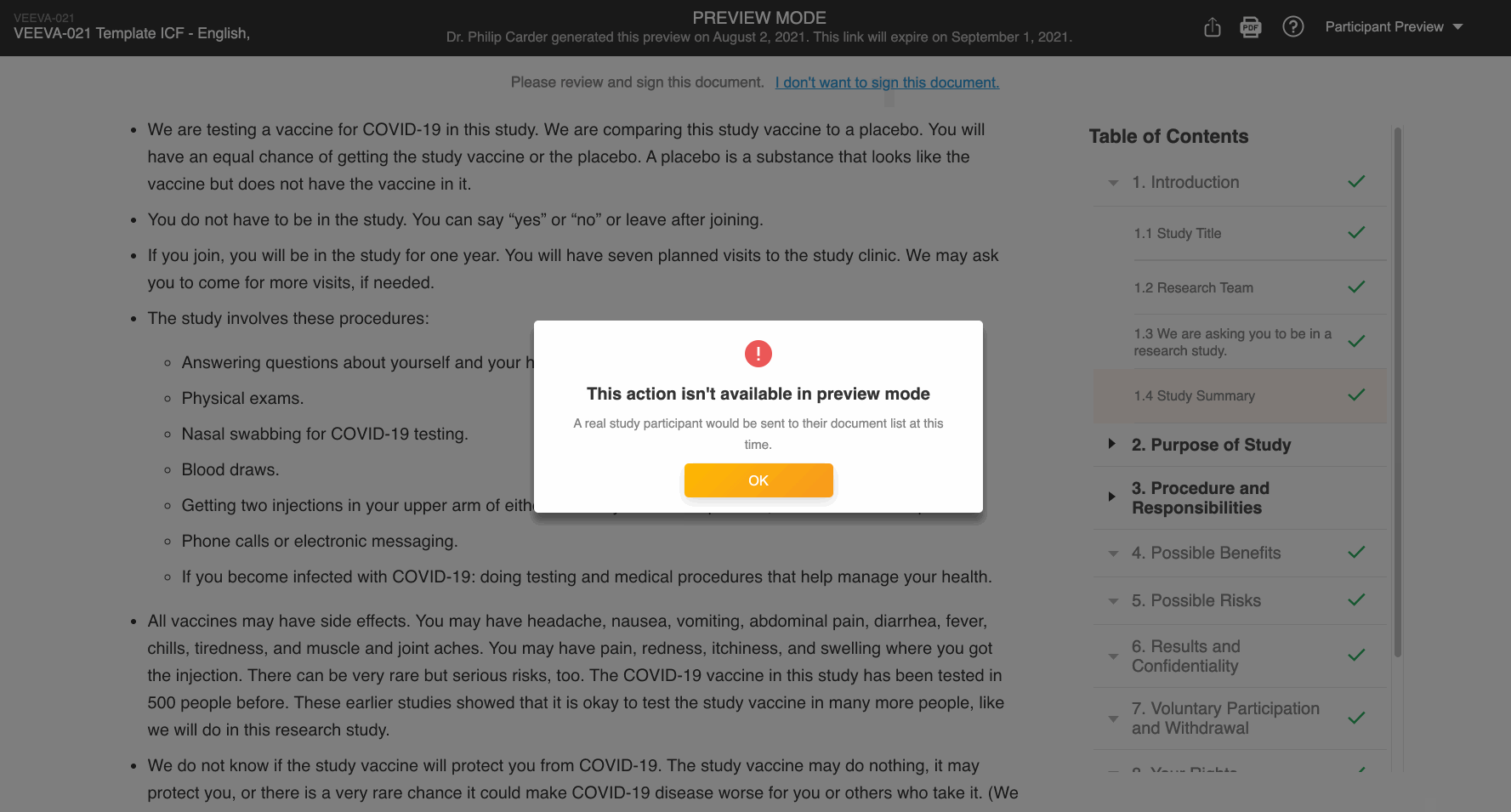
This feature allows Vault users to copy and share preview links of the patient experience of an eConsent form. This enables users to more easily obtain IRB approval of the form. External users can view the preview links without a Vault or MyVeeva for Patients account, and the links expire after 30 days.
Additionally, users in the Veeva eConsent editor can preview their edits from the editor to better understand how the form will be displayed to participants.
Additional Enhancements
The following general enhancements are now available for MyVeeva for Patients and the Veeva eConsent editor:
| Component | Users | Description | Number |
|---|---|---|---|
| eConsent - Editor | Site Staff, Sponsor Staff | When you enter text in a table cell and press ENTER (RETURN) three times, the third line is now single-spaced. Previously, the third line was double-spaced in the editor and .PDF file. | MYVC-984 |
| eConsent - Editor | Site Staff, Sponsor Staff | You can now select the Clear Formatting button on the text editor toolbar to clear all the formatting in a content block or the formatting of your selected text. | MYVC-1448 |
| eConsent - Editor | Site Staff, Sponsor Staff | You can now remove the last content block from a section. Previously, we did not allow empty sections once blocks were added. | MYVC-1644 |
| eConsent - Editor, eConsent - Patient | Site Staff, Participants, Sponsor Staff | When you select or otherwise focus on a content block that is currently offscreen on the table of contents, we now scroll the table of contents to the content block. | MYVC-821 |
| eConsent - Patient | Participants | This feature speeds up the participant's consenting experience by processing the submission in the background after the user submits the document and verifies their identity. This allows the participant to more quickly continue reviewing other documents as necessary. | MYVC-1840 |
| eConsent - Patient | Participants | Documents on the My Documents page are now sorted by the date you completed them on within each study. Previously, the documents were sorted by their names. | MYVC-1054 |
| eConsent - Patient | Participants | We updated the style of tooltips to make them easier to read when you position your mouse's pointer over an item or press an item on a touchscreen. | MYVC-454 |
| Infrastructure | Site Staff, Participants, Sponsor Staff | This feature enables sites, sponsors, and patients to use MyVeeva for Patients in specific regional instances and store data in locations that are legally appropriate for where they operate or live. Currently, we support the US and EU regions. The Veeva eConsent editor uses the region configured for the vault, and MyVeeva for Patients uses the region of the vault from which the eConsent forms are sent. Patients can have accounts in multiple instances, are directed to the regional domain from email invitations, and are redirected to the regional instance associated with their account if they begin to log in to the global domain. | MYVC-1909 |
| Patient UI - General | Participants | You can now view the MyVeeva for Patients terms of service and privacy policy in Spanish. To do so, set Spanish as the first preferred language in your browser settings. | MYVC-1309 |
Fixed Issues
This general release currently includes resolutions to only uncommon or minor cosmetic issues. See the MyVeeva for Patients Known Issues page for issues that participants and staff may still encounter in MyVeeva for Patients and the Veeva eConsent editor.