Workflows and state changes are used to update document statuses, certify documents as copies, or obtain electronic signatures on documents. Workflow tasks that are assigned to you are displayed in the My Tasks view in the SiteVault’s Home tab.
Review the Document Types Spreadsheet to determine which workflows are available for each SiteVault document type.
Document Workflows Overview
Below the table you can find more information on each workflow as well as links to inidividual workflow help pages and supplemental information.
| Workflow | Description |
|---|---|
| Send for eSignature Approval | Collect eSignatures on documents requiring one or more signatures for approval. Not intended for PI oversight. |
| Send for eSignature | Available on document types that require an eSignature for a specific reason (typically noted on the form). Ex. CV, Financial Disclosure, and Informed Consent Form (signed). |
| Send for Investigator eSignature | Send the document to one or more of your study investigators for eSignature. Available on document types that require PI eSignature (typically noted on the form). Ex. 1572 or equivalent, Acceptance of IB, Delegation of Authority, and Protocol Signature Page. |
| Send for Read and Understand | Send one or more study documents to study team members. Each recipient must confirm they have read and understood each study document. |
| Send for Review | Send one or many documents to colleagues who can provide feedback using the annotation tool. |
Initiating Workflows
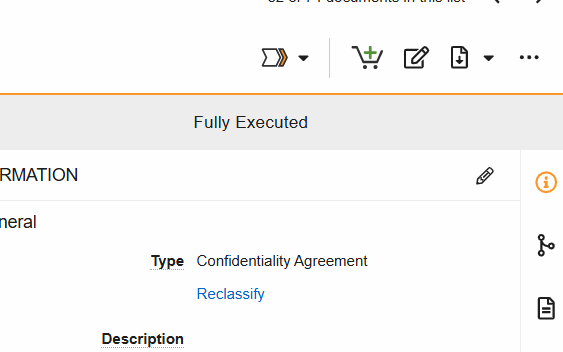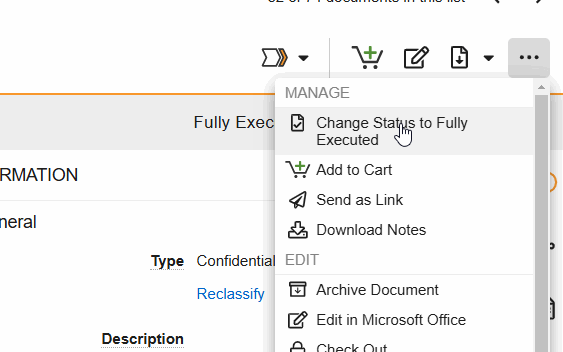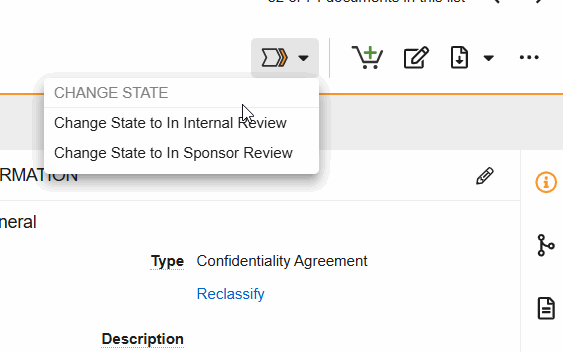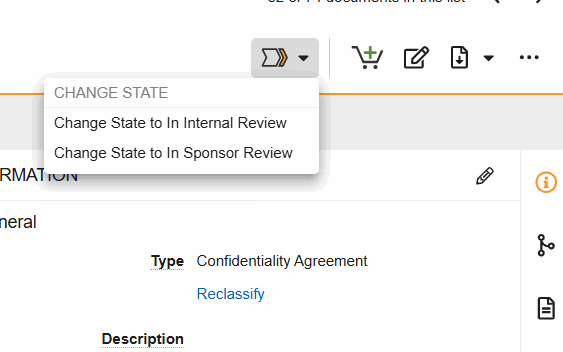
Workflows are initiated from the Document Viewer > Workflow Actions menu (see image) or from the Document Actions menu (…) in the Site and Study eBinders.
eSignature Workflows
The eSignature workflow in SiteVault enables clinical research sites to electronically sign regulatory and study-related documents in compliance with FDA 21 CFR Part 11 and GCP requirements. This eliminates the need for wet ink signatures and manual document handling.
Key Benefits of Adoption
- Regulatory Compliance: Fully compliant with electronic signature regulations.
- Efficiency: Reduces turnaround time for signed documents.
- Audit Readiness: Provides a clear, trackable signature history.
- Remote Accessibility: Enables signing from any location, reducing delays.
Note The electronic signature in SiteVault is nonbiometric and requires the application of two distinct components (a user ID and password) and is compliant with the Food and Drug Administration's (FDA's) 21 CFR Part 11 section §11.200 requirement of electronic signatures that are not based upon biometrics.
Want to learn more?
How to Use eSignatures in SiteVault
eSignature Feature Summary
Starting and Completing Multi-Document Workflows
Review Workflow
The Review workflow allows site staff, monitors, and other stakeholders to review and comment on documents within SiteVault before they are finalized. This ensures accuracy and alignment with study requirements.
Key Benefits of Adoption
- Streamlined Collaboration: Enables real-time feedback and reduces document revisions.
- Improved Accuracy: Ensures documents meet study and regulatory requirements before finalization.
- Reduced Delays: Eliminates back-and-forth emails and manual review processes.
Want to learn more?
How to Use the Review Workflow
Starting and Completing Multi-Document Workflows
Read and Understand Workflow
The Read and Understand workflow ensures that site staff acknowledge and understand critical documents, such as SOPs and study protocols. It tracks completion and provides audit-ready documentation of compliance.
Key Benefits of Adoption
- Improved Compliance: Ensures staff are aware of and understand essential documents.
- Audit Readiness: Provides a clear record of acknowledgments.
- Efficiency: Automates tracking and reminders to prevent delays.
Want to learn more?
How to Use the Read and Understand Workflow
Read and Understand Feature Summary
Starting and Completing Multi-Document Workflows
SiteVault Document State Changes
- Change Status to (final state): Progress the document to its steady state. This state change includes the option to perform Copy Certification.
- Change State to Internal Review: Indicate that the document is in internal review.
- Change State to In Sponsor Review: Indicate that the document is in sponsor review.