SiteVault Release Highlights
SiteVault General
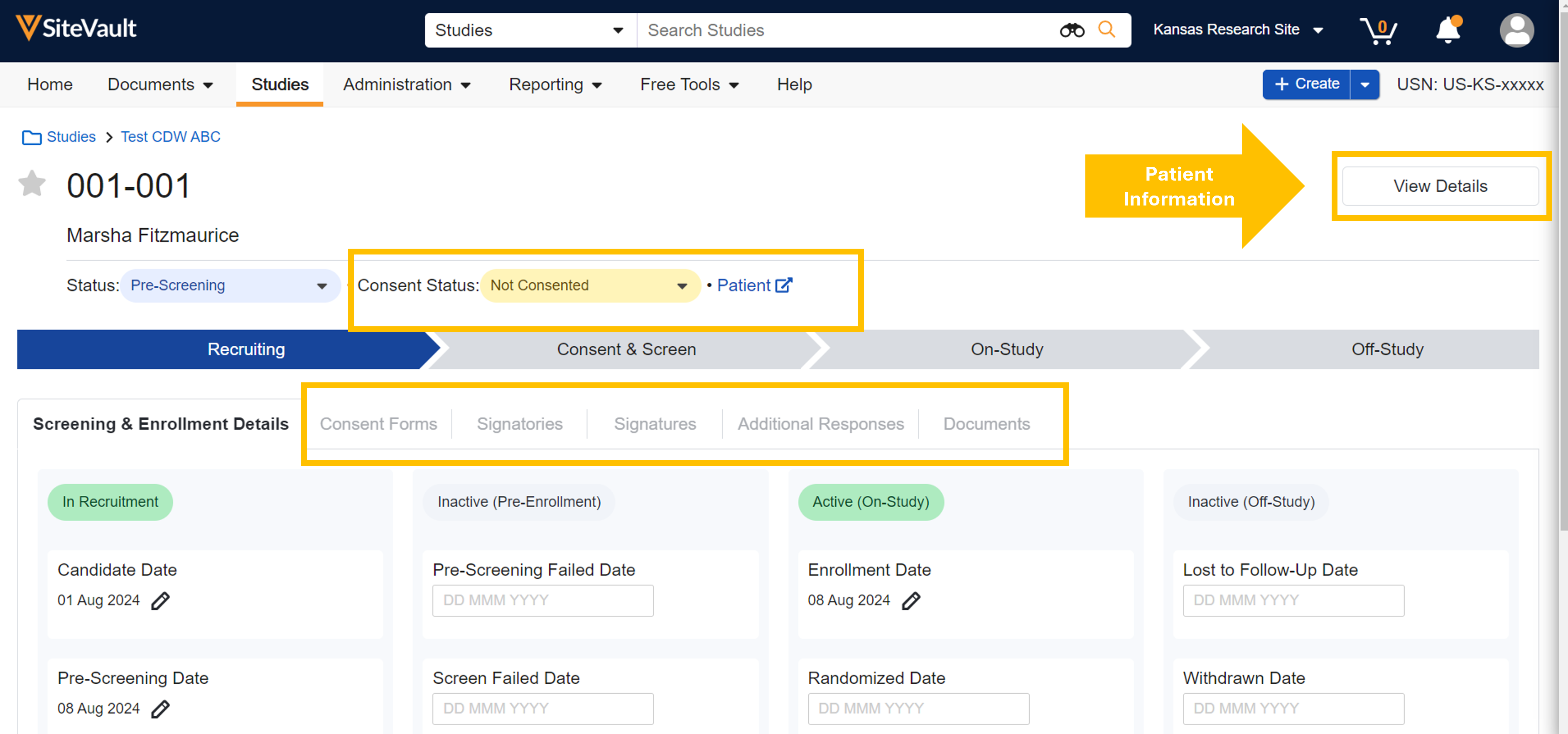
Study Details Page
The Study Details page has shifted to a horizontal tab presentation to simplify navigation. Key information remains available on the main Study Details page, while items accessed less often are in the View Details panel. Review these changes and more in the highlights below.
- Key study information, such as the PI and sponsor, is prominently placed just below the study name for quick access.
- The eBinder link opens the Study eBinder, already pre-filtered for the study.
- A horizontal menu of standard tabs offers simplified navigation to Teams, Participants, Monitors and auditors, Organizations, and Products.
- Studies enabled with eConsent or Digital Delegation Log may include additional tabs such as Consent Forms (for managing blank consent forms) and Responsibilities (for managing Digital Delegation responsibilities).
- The View Details button opens a panel of additional study information, including three action fields for updating the study status and enabling/disabling eConsent and Digital Delegation Log.
- Specific study needs can trigger an attention tab to alert users to complete a task. For example, when a study enabled with Digital Delegation Log has delegations to send for PI approval, the first tab of the study is labeled “PI Approval”, and the task details are provided.
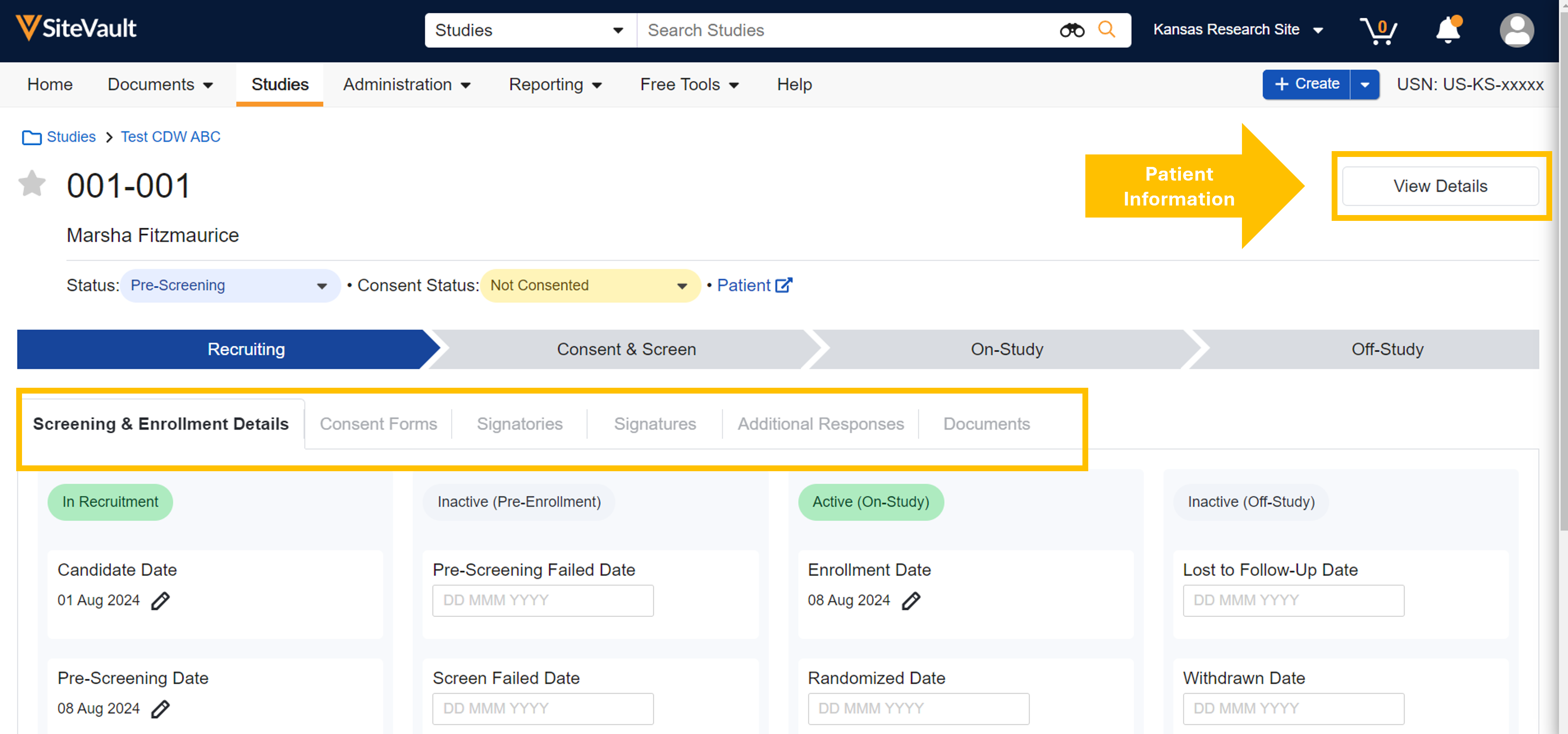
Participant Details Page
The Participant Detail page allows users to easily view participant progress and apply study actions, including eConsent actions. The View Details button opens a panel with additional participant information and actions. This enhancement introduces the ability to manage Patient and Participant record details from the same page, reducing navigation. Review these changes and more in the highlights below.
- When a Patient record is associated with the Participant record, the patient name is displayed and a link to the Patient record is provided.
- The Status dropdown allows you to update the participant’s status to any listed state. When selected, key states will prompt you to assign a date to associate with the state change. Selecting a date is an optional action.
- The Consent Status field is available for viewing or updating.
- A horizontal menu of standard tabs offers simplified navigation to Screening & Enrollment Details, Consent Forms, and Documents.
- When eConsent is enabled on a study, the horizontal navigation includes additional tabs such as Signatories, Signatures, and Additional Responses.
- The View Details button opens a panel of additional Participant and Patient record information, including two action fields for updating the participant’s Status and Consent Status (also available on the Participant Details page). The Add/Update Patient link allows you to associate a Patient record with the Participant record.
Monitor/External User Creation - Override Option Removed
The Override Option link that was sometimes displayed when creating a new Monitor/External User is no longer available. This link was offered to provide an option for users who wanted to avoid reusing their existing Vault accounts. Instead, we recommend the Monitor/External User self-register for VeevaID before an administrator adds them to their site.
Site Documents Enhancements
These updates offer site users more flexibility when working with Site Documents.
- Administrators can download the document source in all states.
- Users with document access can download a viewable rendition in all states.
- Users with document access can cancel an active Read & Understand workflow and the document will remain in the current version.
- Users with document access can download documents with the signature page attached (if signed with SiteVault eSignature).
- Users with document access can transition a draft document to Effective without requiring an eSignature.
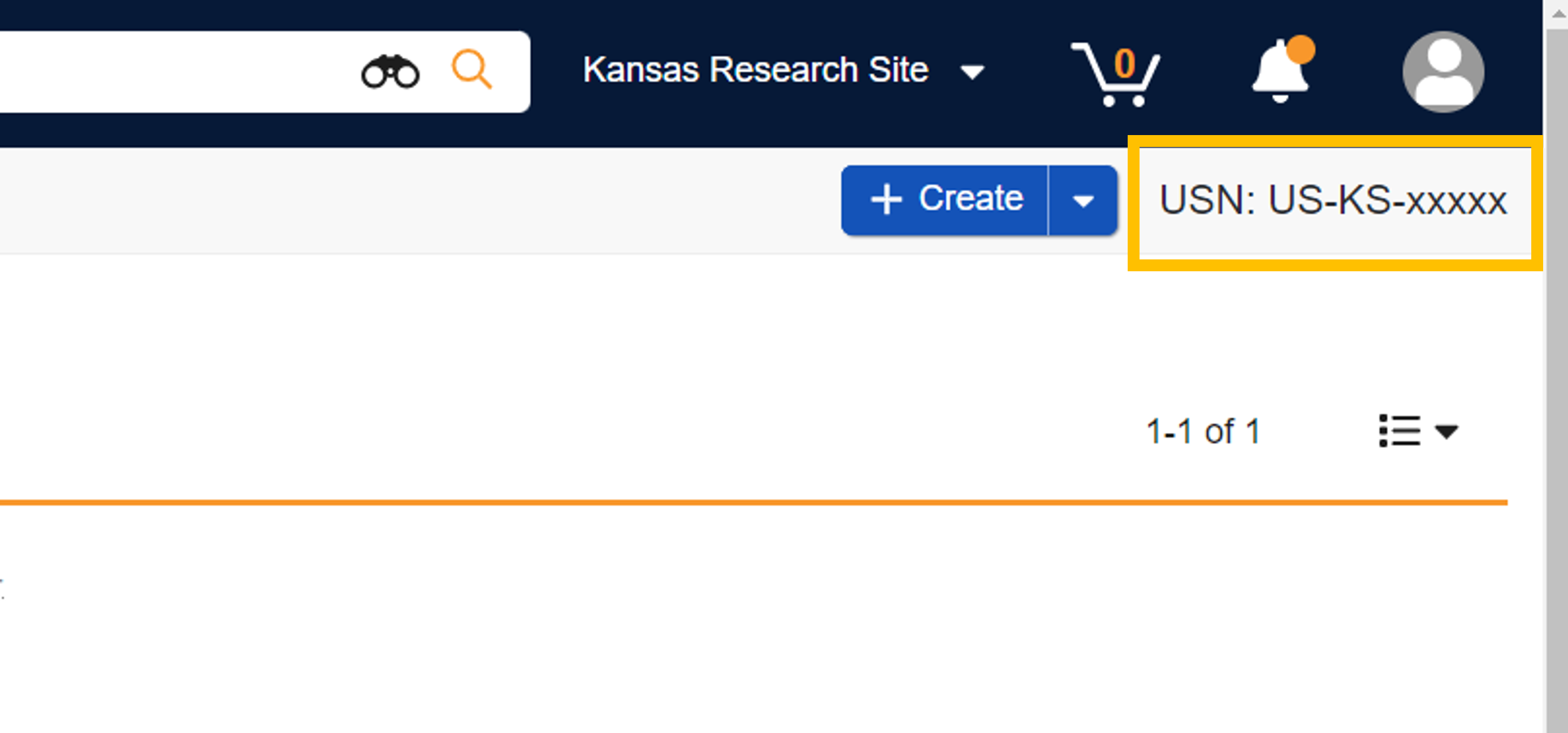
Accessing the Universal Site Number
The Universal Site Number (USN) is displayed at the top right of your screen, below the profile button. It is available when accessing in the site context.
SiteVault eConsent
Site users will enjoy an enhanced Veeva eConsent experience with minimal navigation and increased functionality. eConsent activity is seamlessly integrated into the highly accessed areas of a study in eISF. When enabled on a study, eConsent actions are available from the Study eBinder and the Study and Participant Detail pages. Review these changes and more in the highlights below.
eConsent on the Study Details Page
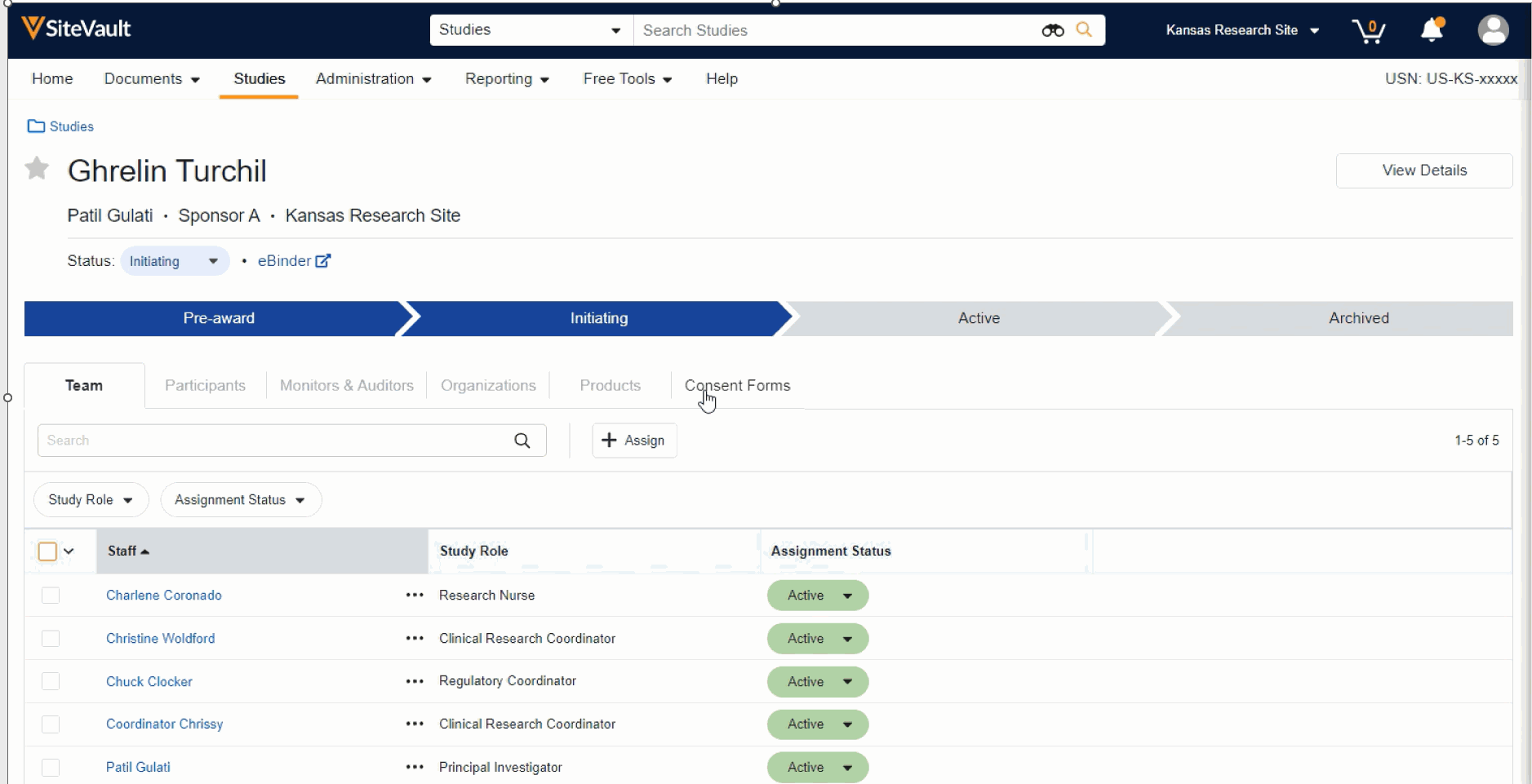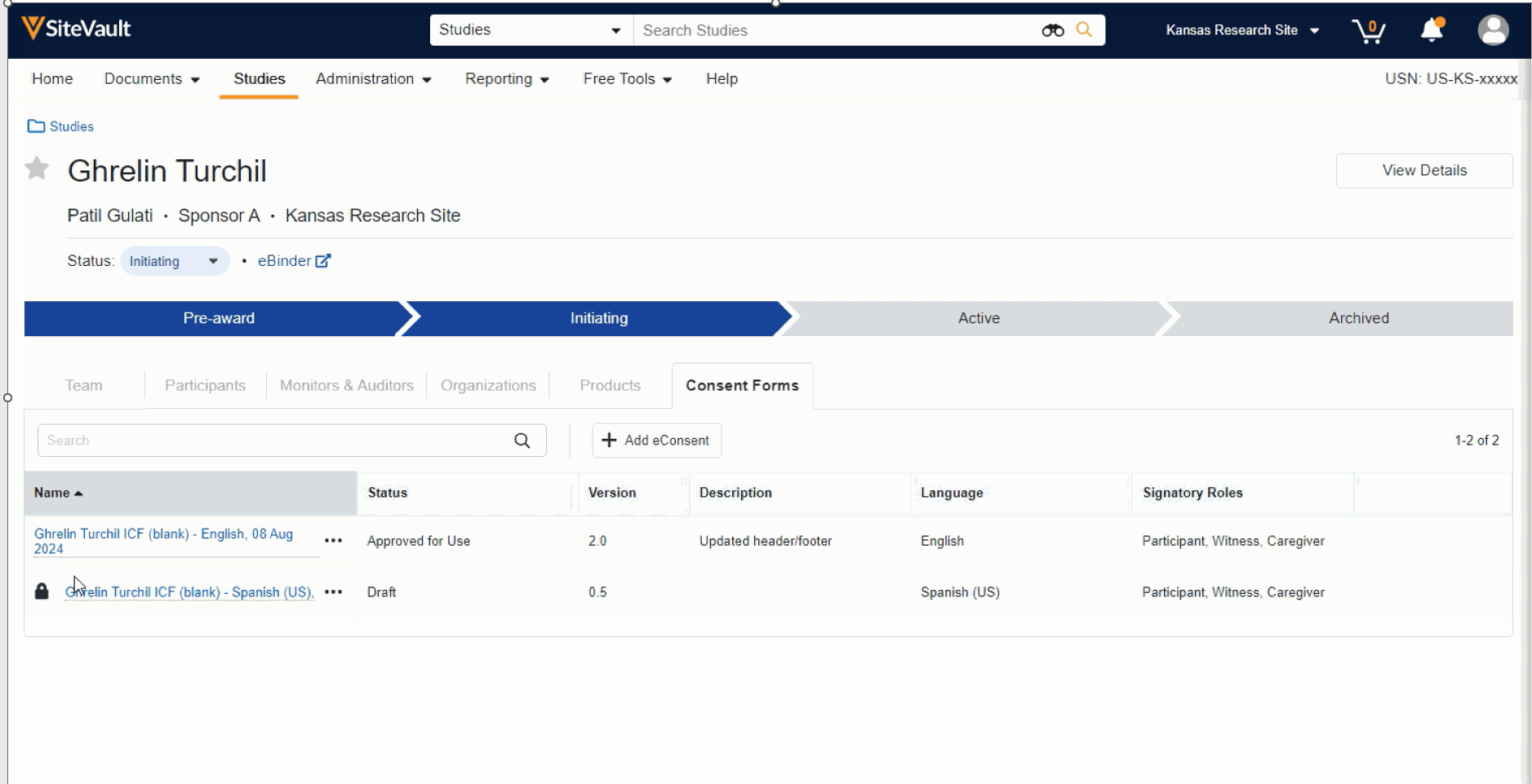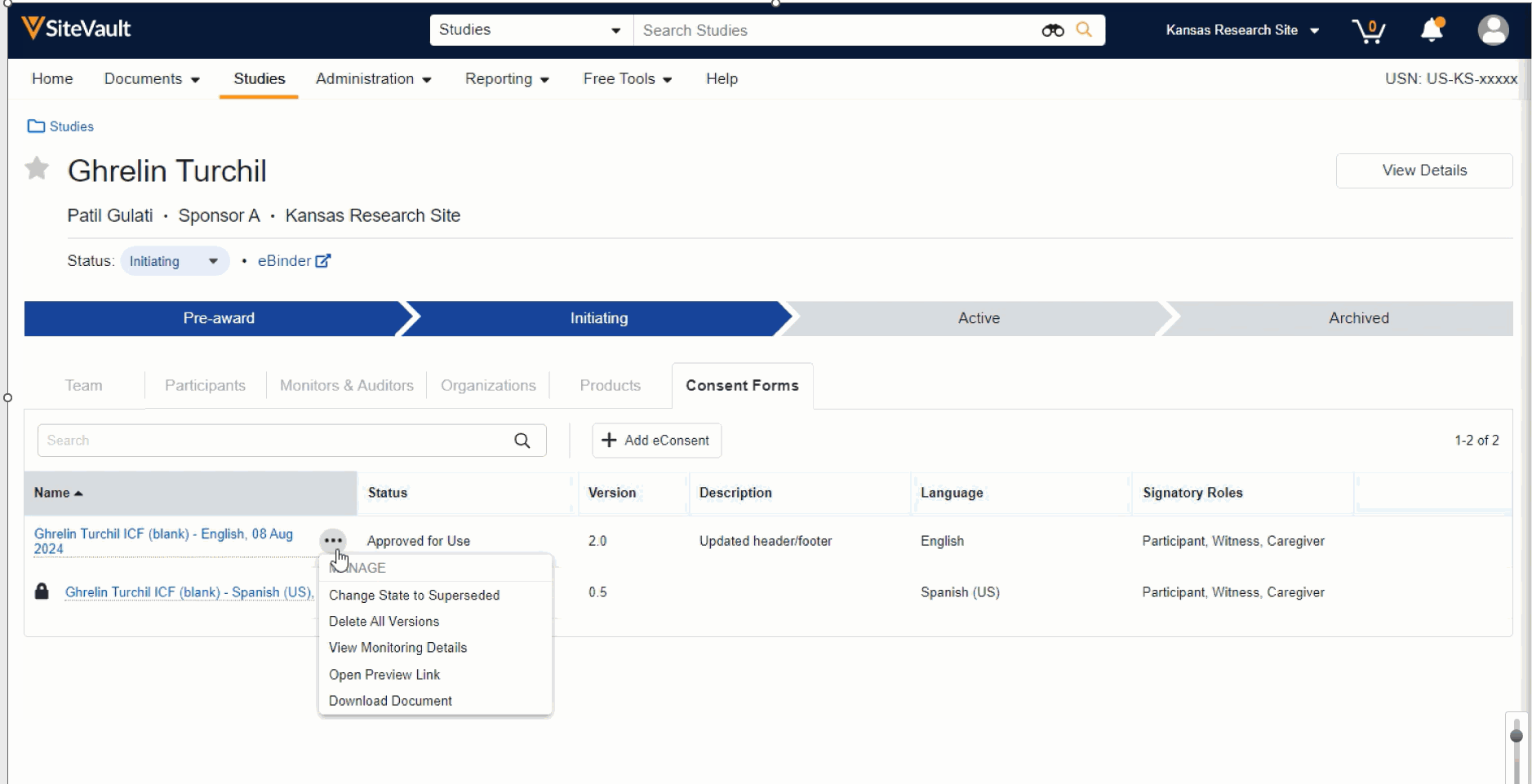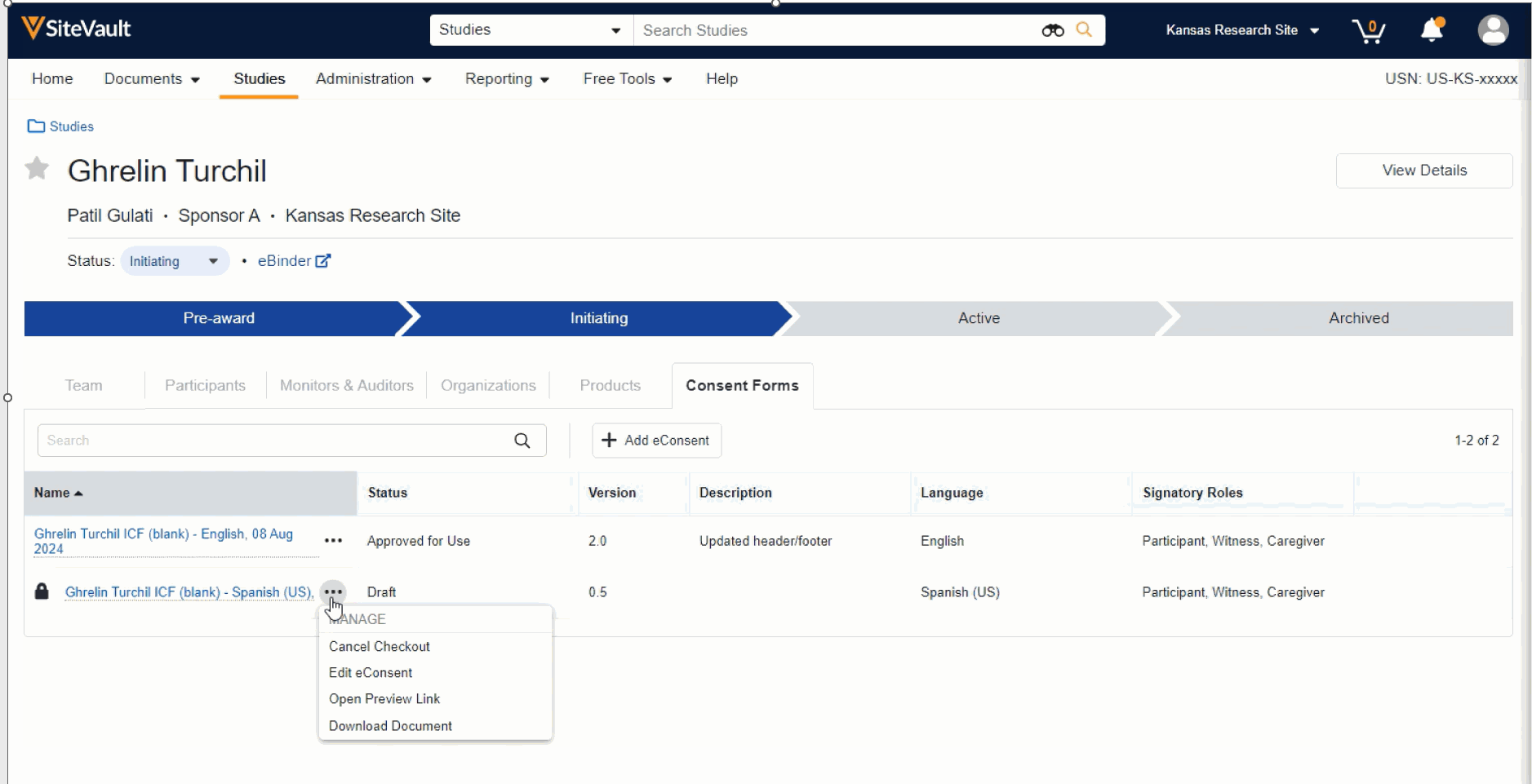
- The Consent Forms tab provides a study-level overview of all blank consent forms, their version, status, description, and more. From this view, you can:
- Add a new form by selecting the + Add eConsent button
- Manage (edit, approve, send, preview, etc.) an existing consent form by selecting the associated Document Actions menu. The option to approve an eConsent form is available once the necessary edits have been completed.
- Confirm if an eConsent form is checked out for editing by noting if the lock icon is present.
- Administrators can easily enable or disable Veeva eConsent on a study by updating the eConsent field in the View Details panel, on the Study Details page.
- eConsent can also be enabled or disabled on a document-by-document basis if you’re piloting eConsent with a subset of study forms.
eConsent in the Study eBinder
When working on an eConsent study in the Study eBinder, the Document Actions menu offers the following eConsent actions:
- Approve and Send
- Create Draft (eConsent)
- Send to Sponsor
eConsent on the Participants Tab
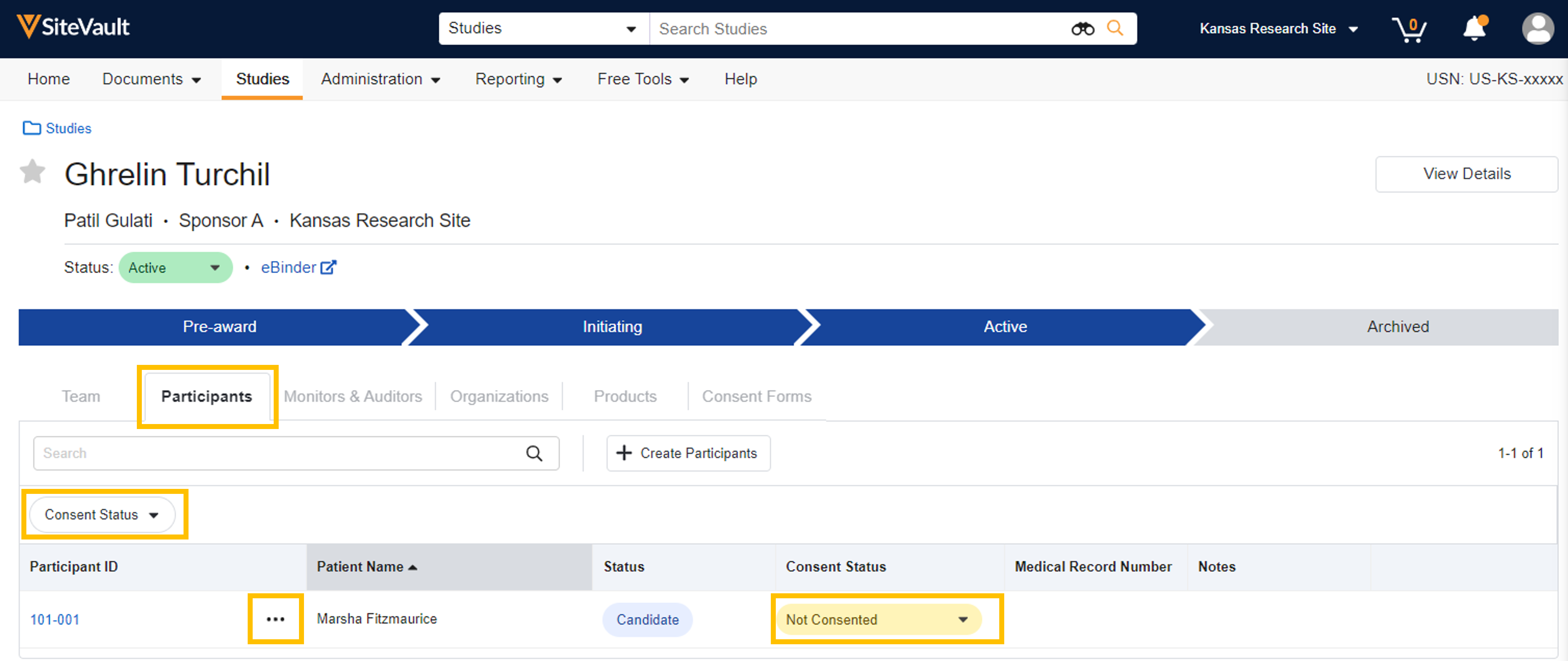
- The Consent Status dropdown is available for filtering the participant list.
- The Consent Status field is an automated field based on eConsent signature activity. Site users can update the field manually when needed.
- Participant records include an Actions menu that provides eConsent actions such as Create Signatory, Generate Activation Code, and Start Consent.
- The Start Consent action initiates the consent activity for both remote and on-site consenting.
eConsent on the Participant Detail Page
Select a participant from the Participant tab to access the Participant Details page, where you can view and update the participant’s eConsent information.
- The Consent Status field is available for viewing or updating.
- eConsent-enabled studies include the following eConsent tabs and related actions on the Participant Detail page:
- Consent Forms: Review or manage consent forms for the selected participant
- Signatories: Add, edit, or delete participant signatories
- Signatures: Review signature responses from this participant and their signatories
- Additional Responses: Review responses to consent form questions
- Documents: Review documents related to the selected participant
Two Witnesses on eConsent Forms
A maximum of two Witness signatures can be added to eConsents where multiple Witnesses signatories are needed.
Connected Study Invitation and Acceptance
This feature introduces an eConsent study invitation process that streamlines the invitation to connect, site acceptance, and study creation. When a sponsor sends the first document from their Clinical Vault to the site, the invitation to connect on the study is initiated. Administrators are assigned an eConsent Study Acceptance task to review the invitation details. Once an administrator accepts the request to connect, the documents are delivered to SiteVault. As part of this change, all eConsent forms are sent to the connected Clinical Vault on approval, whether they are sponsor- or site-originated.
For more information, see eConsent Help.
SiteVault eISF
Monitoring Enhancements
Monitor Experience
Updates to the Monitoring Process
For monitors, the process for reviewing documents and logging issues has been updated to support new functionality in this release. In addition, the process has been simplified and streamlined to make it easier to learn and use.
- Added ability to monitor Profile Documents: In addition to study-specific documents, monitors can review profile documents (CVs, training, etc.) and assign a monitoring status for each document as it applies to each study.
- Viewing/Editing Monitoring Status: When viewing a document from the Document Viewer, monitors can select the View Monitoring Status option from the Document Actions menu to review or update the document’s monitoring status across all applicable document versions and studies. Monitoring Status values have changed:
- Ready for Review: The document is in its steady or final state and is ready for monitoring review.
- NEW Review Not Required: The document is in its steady or final state and does not require review.
- Reviewed, No Issues: The document has been reviewed and no issues were found.
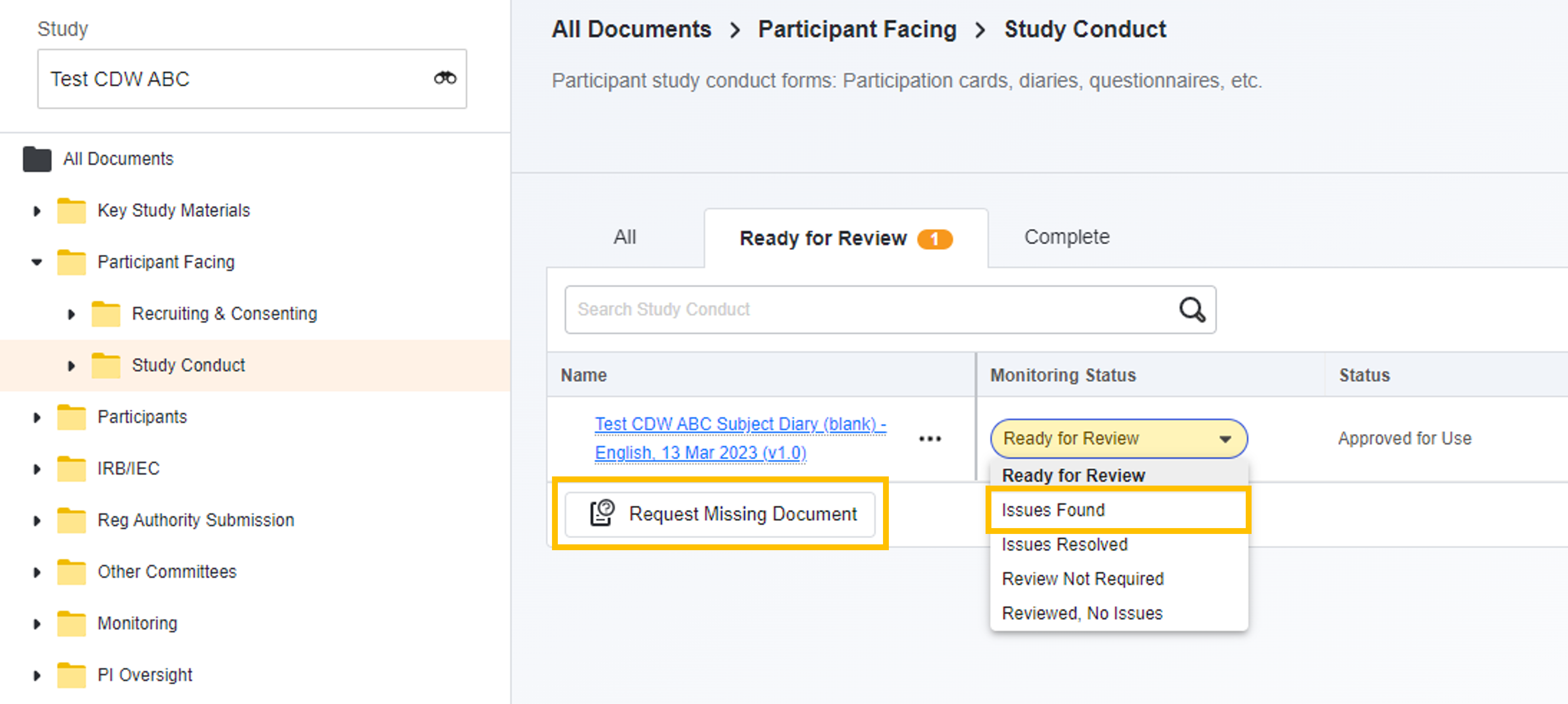
- Issues Found: The monitor has initiated the process to log a monitoring issue related to the document.
- Issues Resolved: Indicates that any logged issues have been resolved by the site staff and are awaiting the monitor’s review of the resolution details.
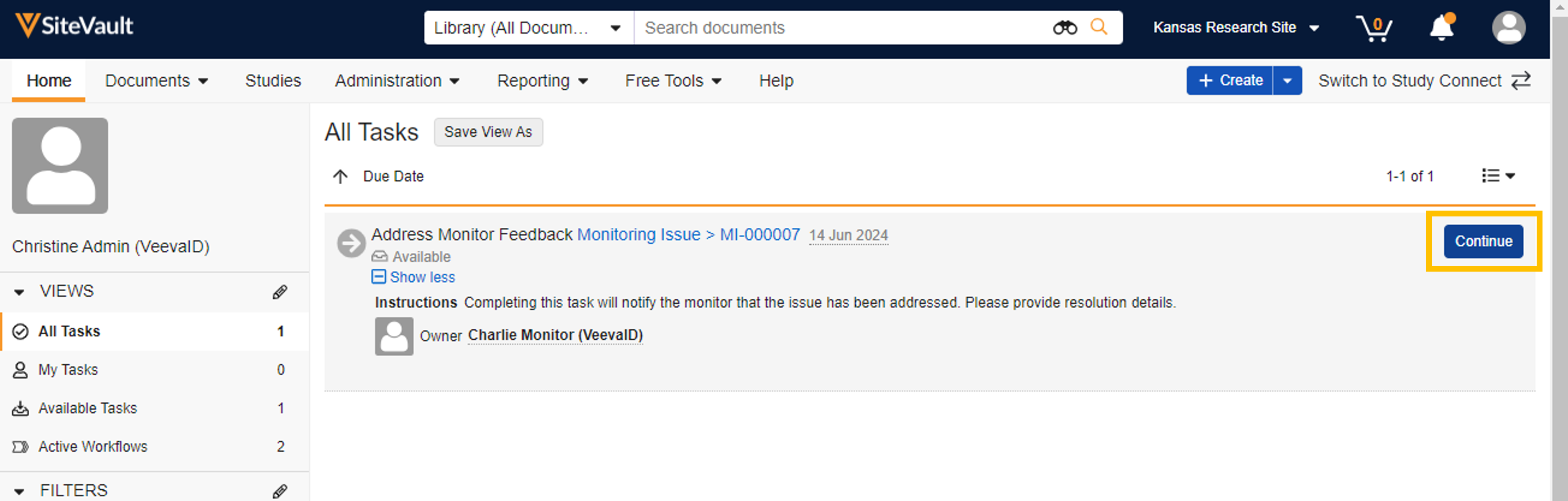
- Issue Logging: When the Issues Found monitoring status is selected for a document, the monitor is prompted to provide the details of the issue. Once the issue is logged, site users assigned to the study are assigned a task to address the issue. Once the task is completed (by the accepting site user), the monitor is notified.
- Request Missing Documents: When browsing eBinder, monitors will see a Request Missing Documents button. When selected, the monitor is prompted to provide the details of the missing document, then an issue is logged. Once the issue is logged, site users assigned to the study are assigned a task to address the issue. Once the task is completed (by the accepting site user), the monitor is notified.
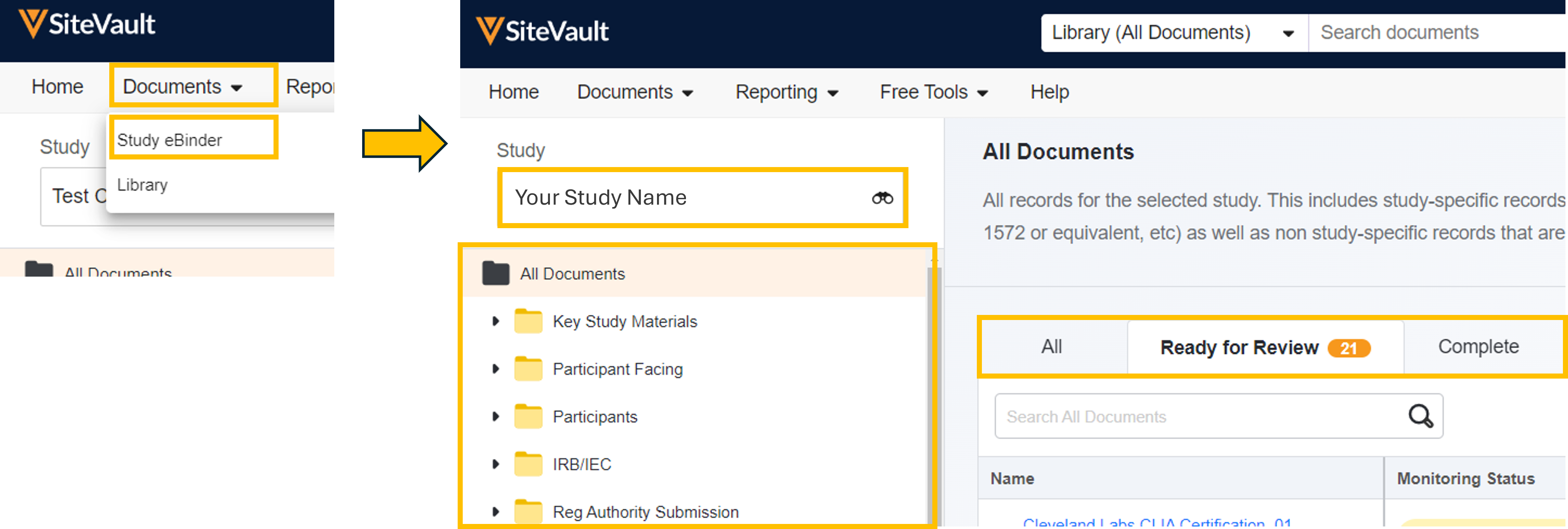
Monitoring in eBinder
For Monitors, the look and feel of the Study eBinder has changed to better support the monitoring process in SiteVault. Monitoring tools are streamlined into the Study eBinder environment, simplifying the monitor-to-site interaction.
- Status Tabs: The monitoring view of the Study eBinder includes tabs labeled All, Ready for Review, and Complete, providing the monitor or CRA a quick snapshot of the current status of study documents.
- Monitoring Status Column: The Monitoring Status column provides an editable status selector to indicate document monitoring progress.
- Open Issues: The Open Issues tab provides the monitor with a view of issues requiring attention (including missing document requests). Selecting an issue will open the issue record, presenting the issue details. Monitors can edit the details of an issue, send the issue to the staff, or cancel the issue (and the associated site user task).
Site Experience
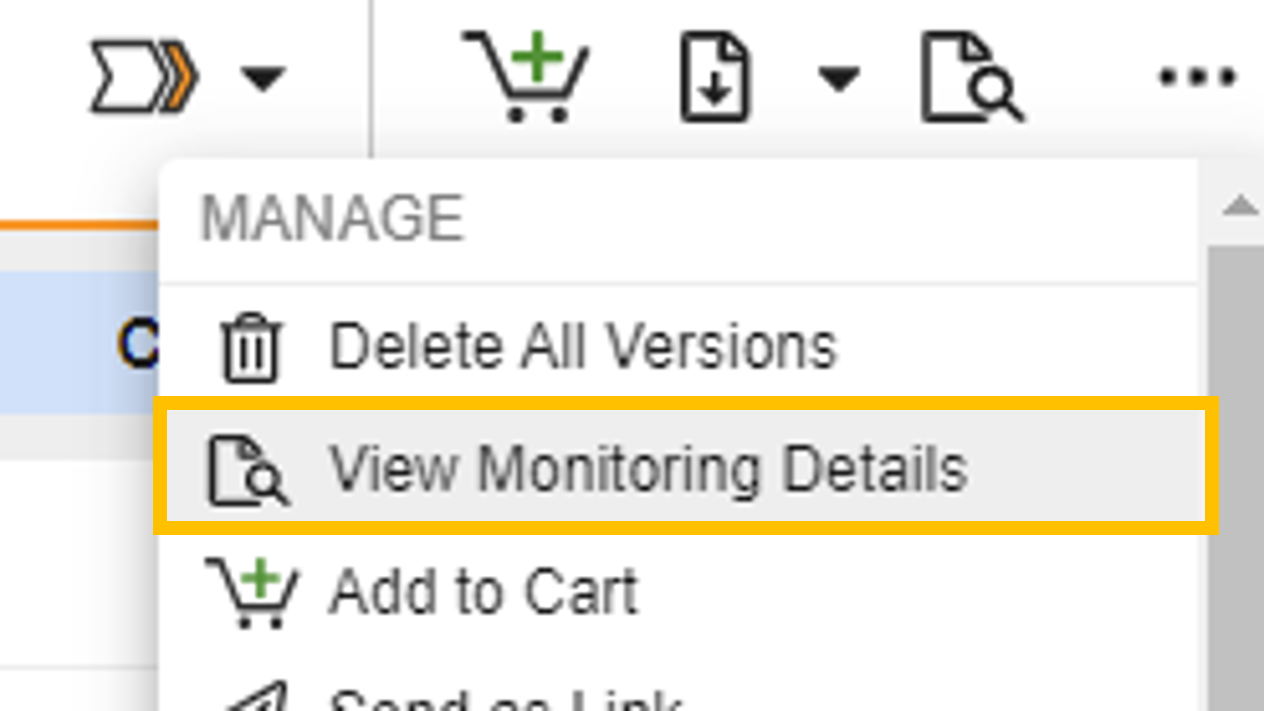
Viewing Monitoring Status
When viewing a document in the Document Viewer, site users can select View Monitoring Status from the Document Actions menu to view the document’s monitoring status across all applicable document versions and studies
Missing Document Requests
Monitors can log an issue to request the site staff locate and/or upload a missing document. Previously, an issue had to be attached to a document in order to be logged.
Resolving Monitoring Issues
When a monitor logs a document issue or requests a missing document, a task is automatically sent to all Site Admins assigned to the study. Once an assignee accepts the task, it will no longer appear as available to the other assigned users. When the task is completed, the monitor is notified. Site users can run a report to track monitoring issue activity.
For more information, see Monitoring Help.
SiteVault Study Connect
Retiring Study Connect
Document Exchange and Safety Distribution have moved out of SiteVault Study Connect and into Veeva Site Connect. Site Connect is a Veeva product owned by sponsors/CROs that allows sponsors and sites to collaborate in one place. Learn more about this shift on the Site Connect Transition page. If you are using Veeva eConsent in Study Connect, our Site Solutions team will be reaching out to introduce you to eConsent in SiteVault eISF.