SiteVault Release Highlights
SiteVault
-
VeevaID in SiteVault
VeevaID provides single sign-on to Veeva systems. All SiteVault users with an “@sitevault.com” user name have been migrated to use VeevaID with the 23R3 release. VeevaIDs will be issued to all newly added Staff and Monitors/External Users unless an existing Vault account is chosen by the administrator who is adding the Staff or Monitor/External User. Enterprise customers can block the use of VeevaID and cross-domain accounts for new External Users.Existing Users
Your accounts are automatically upgraded to VeevaID as part of the release. Watch for a VeevaID email, advising you of your new login process.Logging in with VeevaID
Following the release, log in to SiteVault using your “@sitevault.com” username and current password. Your account will upgrade automatically and you’ll receive a notification that you’ve been converted to VeevaID. Upon subsequent logins, your username will be your email address. Your password will remain the same.Note: If you have multiple VeevaID accounts or share a computer with another VeevaID user, you can select Switch user to enter a different email address.
Additional User Administration Impacts
- Email addresses must be unique within a Research Organization.
- Staff users cannot be added as cross-domain users.
For more information, see Logging In and Out.
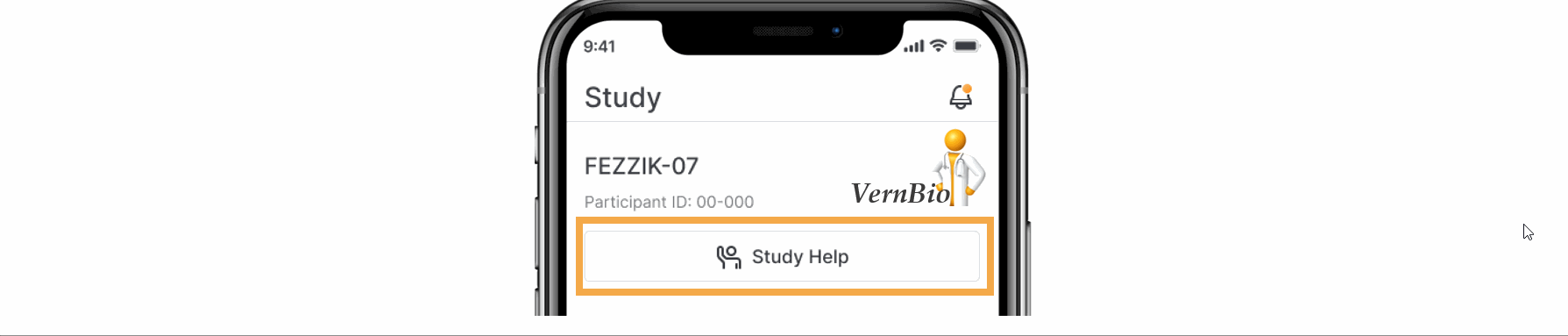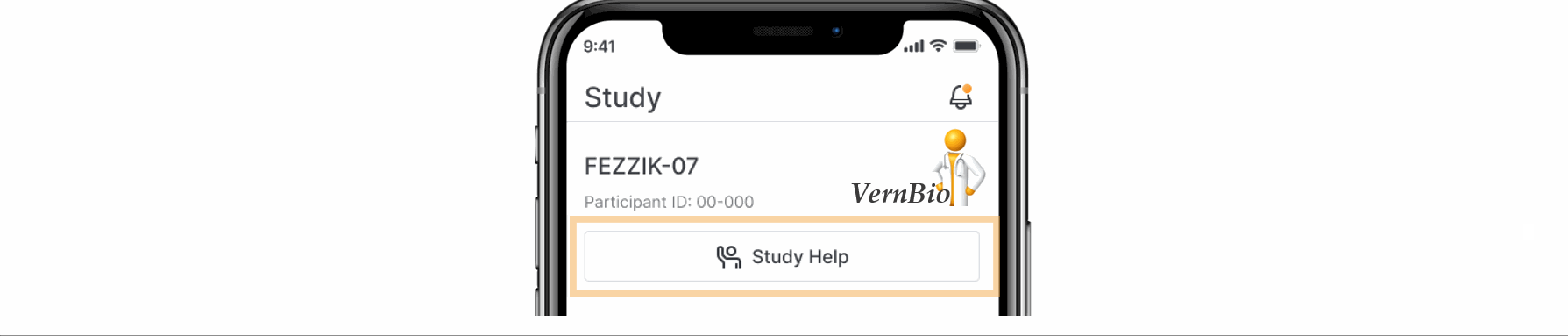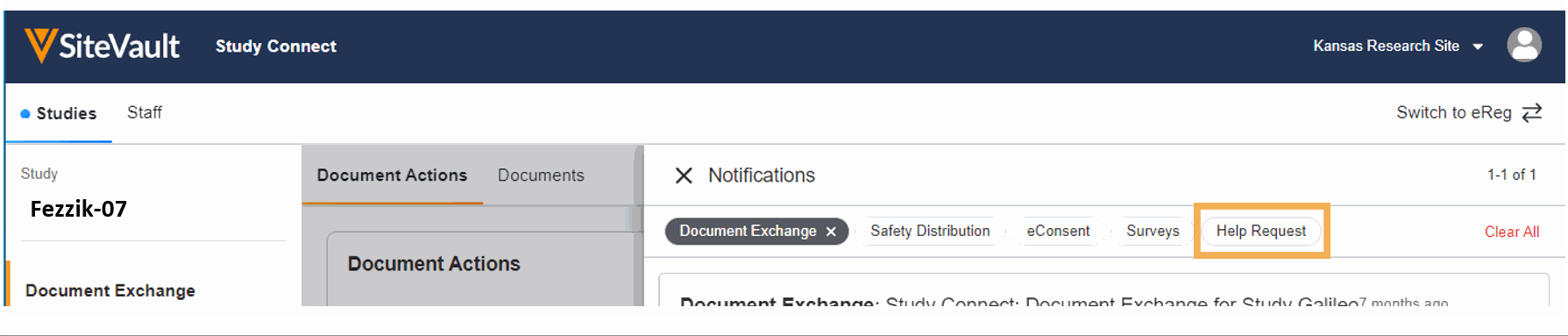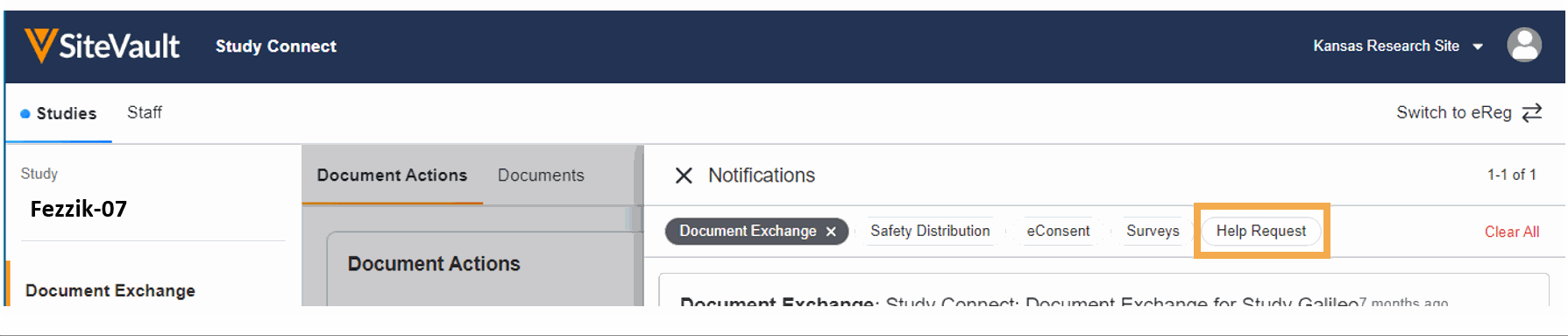
- Ask for Help Request from MyVeeva for Patients
With this feature, SiteVault system and email notifications will include patient requests for assistance submitted through the MyVeeva for Patients application. Notification details include the requestor, the question topic, and the preferred method of contact. The requestor detail includes a direct link to the requestor’s contact information. When the request for help is initiated in MyVeeva for Patients, the requestor is advised “The response will not be immediate, but a team member will contact you as soon as they can.”
SiteVault eReg
Managing Documents
-
Site eBinder
This feature introduces a central location to manage site operational documents not associated with a study. Using the familiar structure and navigation of the Study eBinder, users can upload, access, approve, and initiate workflows on site documents. The site eBinder also offers the ability to bulk upload staff and organization profile documents. Non-study-specific documents filed in the Study eBinder, such as those in the Staff and Partner Organizations folders, are accessible from either eBinder.Site Documents appear in the following eBinder folder structure:
- SOPs & Policies
- Policy Memo
- Standard Operating Procedure
- Work Instruction
- Staff
- Curriculum Vitae (CV)
- Medical License
- Signature and Initials
- Training Evidence (non-study-specific)
- Partner Organizations
- IRbs/IECs
- IRB/IEC Compliance
- IRB/IEC Composition
- Labs
- Lab Certification
- Lab Director Qualifications
- Lab Normal Ranges
- SOPs & Policies
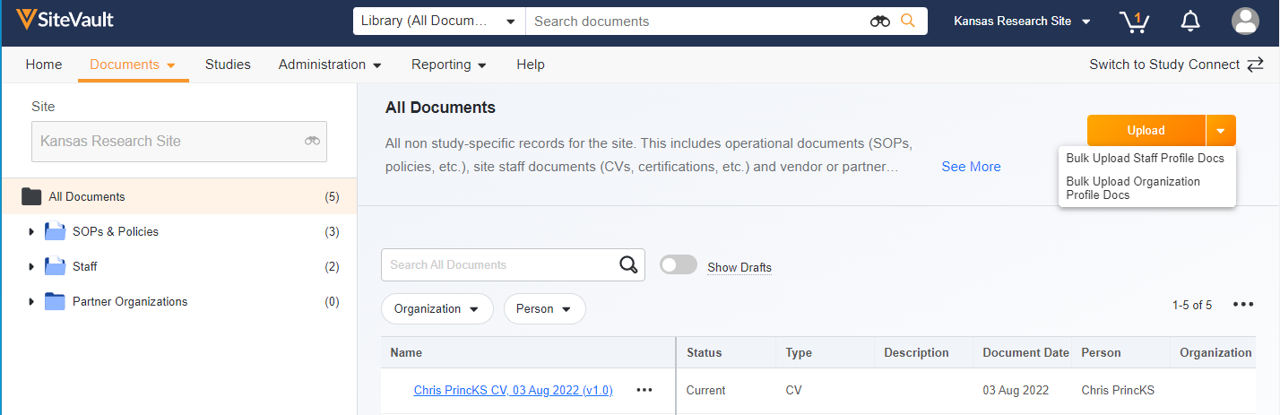
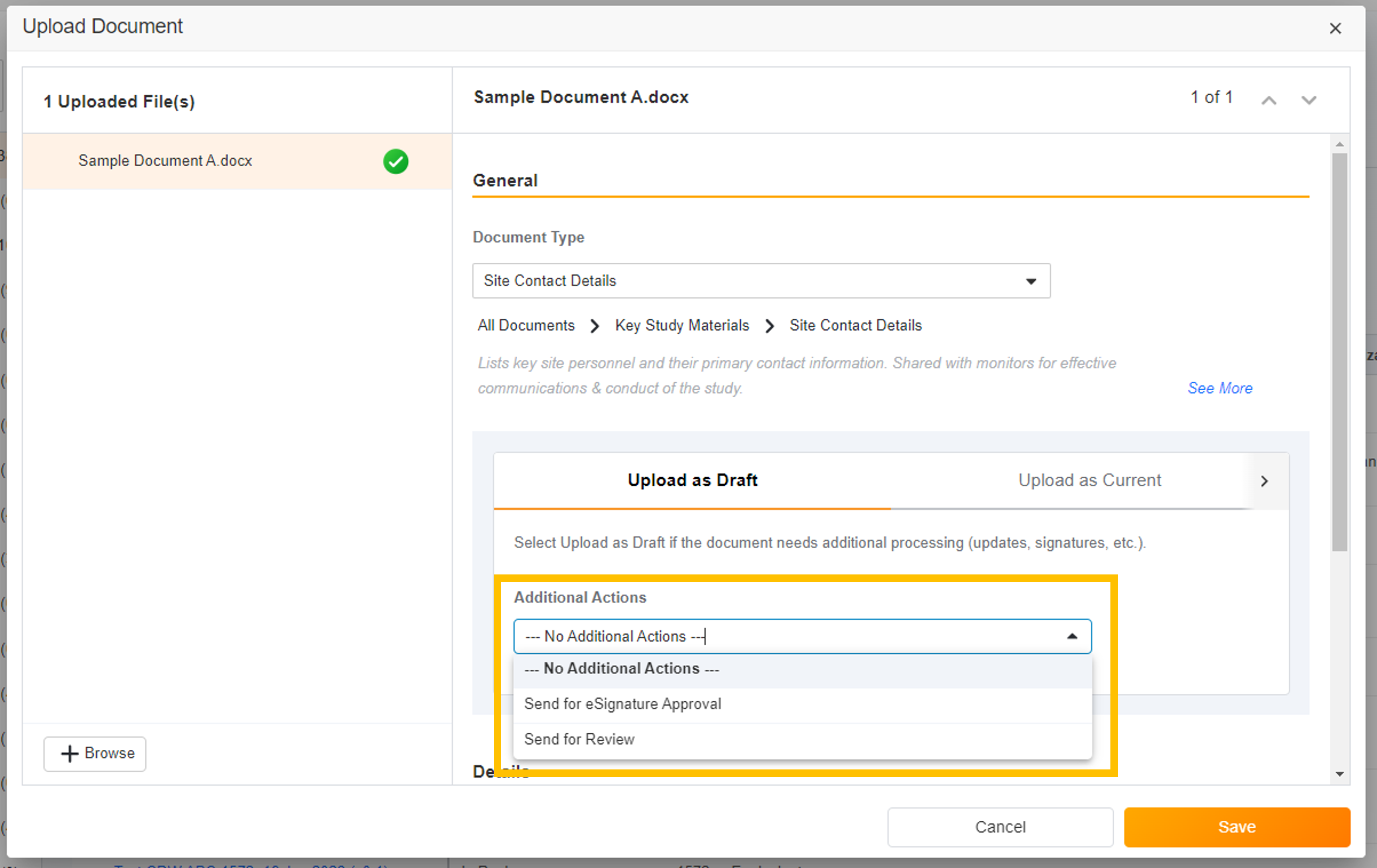
- Upload and Start Workflow
This feature expands the current upload functionality to include the ability to initiate a workflow when uploading to the Study eBinder or Site eBinder. Users can initiate eSignature, Read and Understand, and Review workflows without opening each document after upload.
-
Additional Study eBinder Document Actions
With this feature, actions that were previously only available from the document info page are now available from the action menu in Study eBinder. For sites, newly available actions include Send for Review, Send for eSignature, Send for Read and Understand, and others. For monitors, newly available actions include Passed Review with No Issues and Review Issues Found. -
Document Sharing for Caregivers
This feature allows sites to share non-eConsent documents with caregivers before, during, or after a study.
Managing Users
-
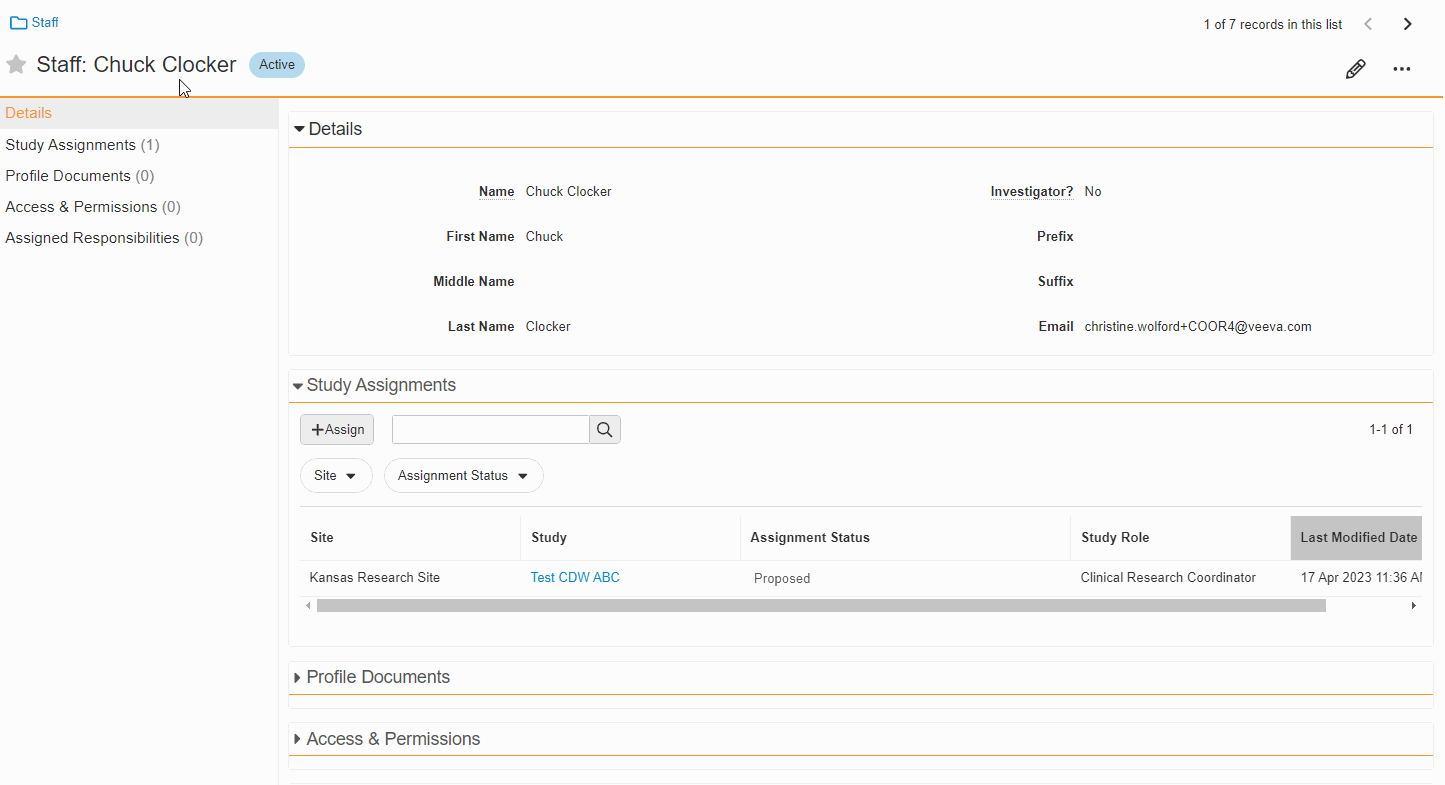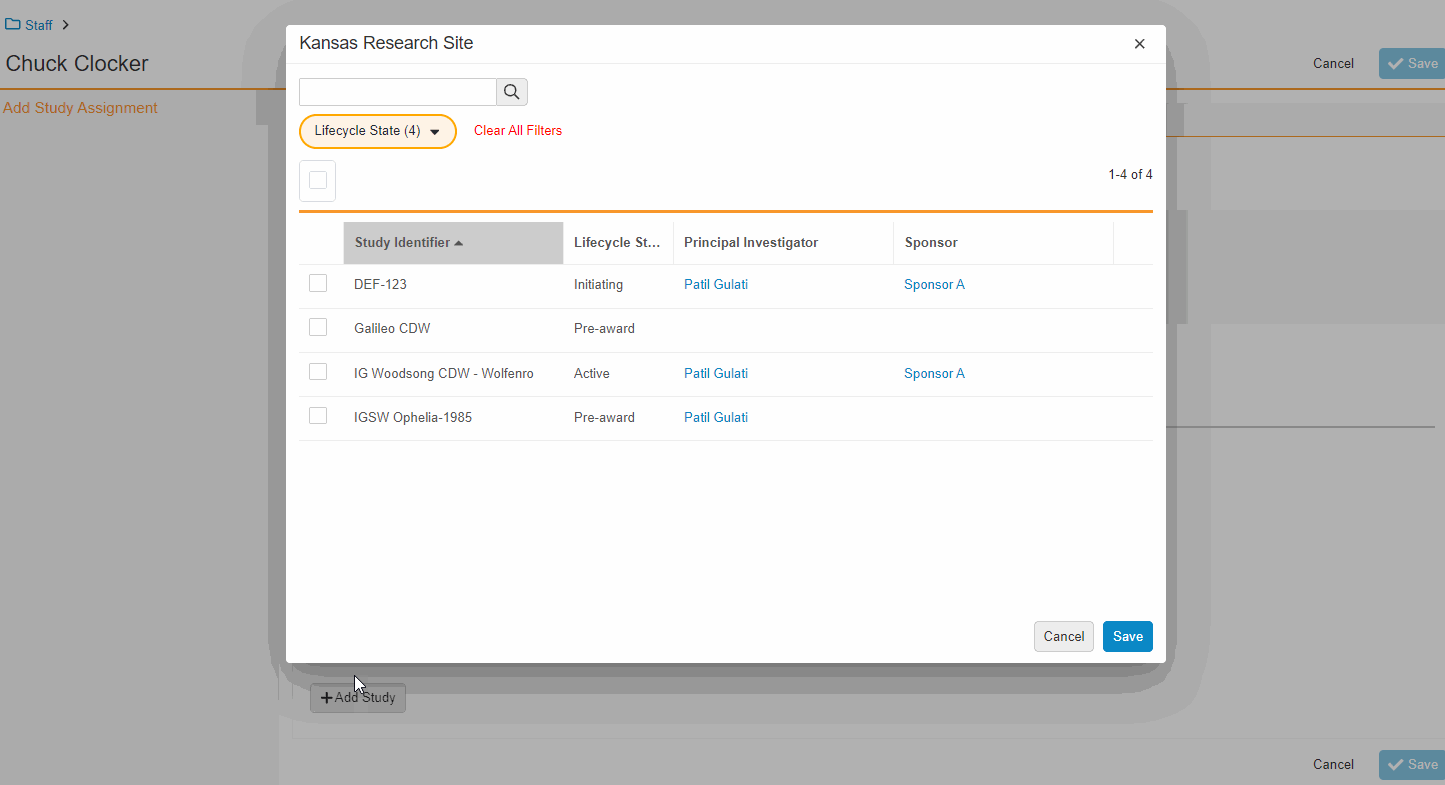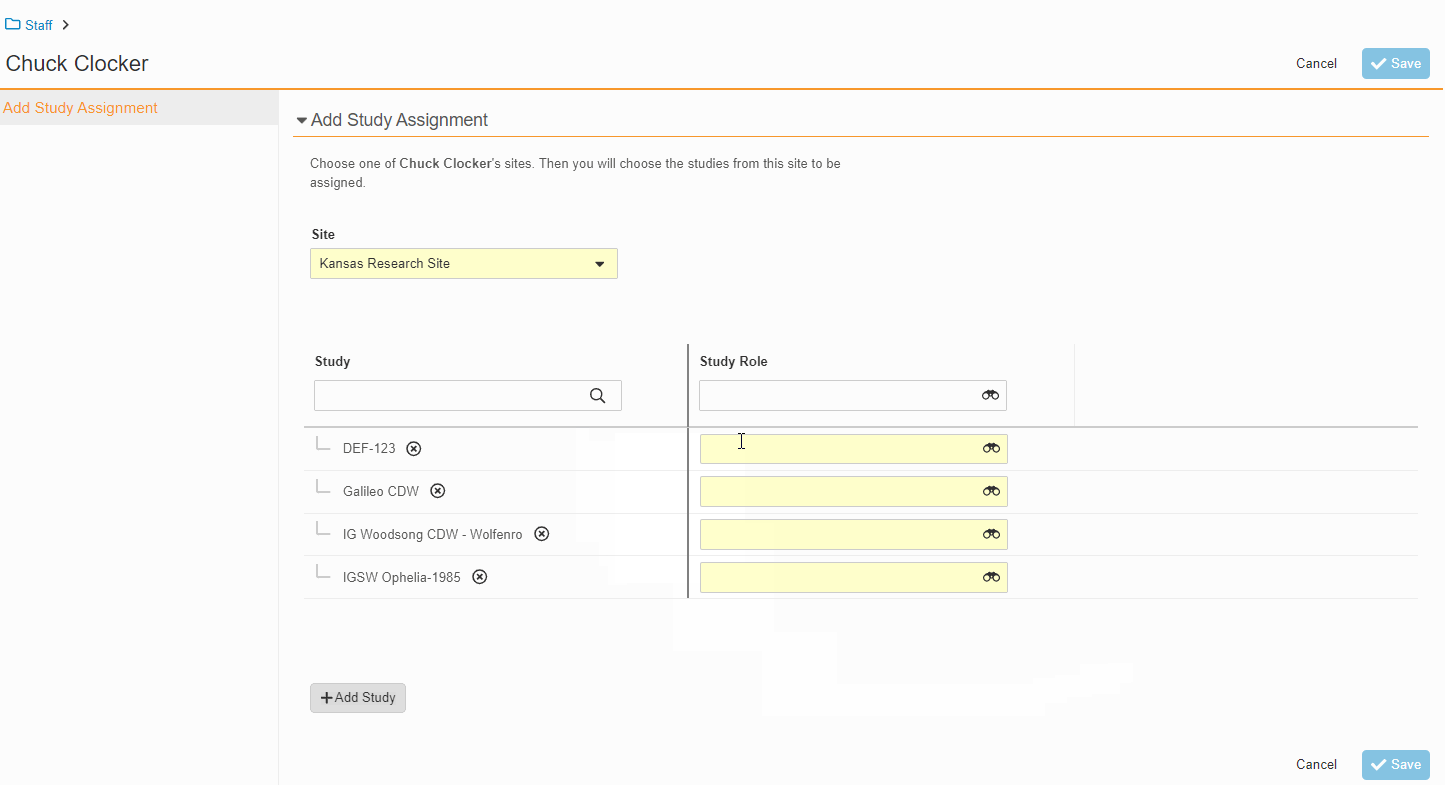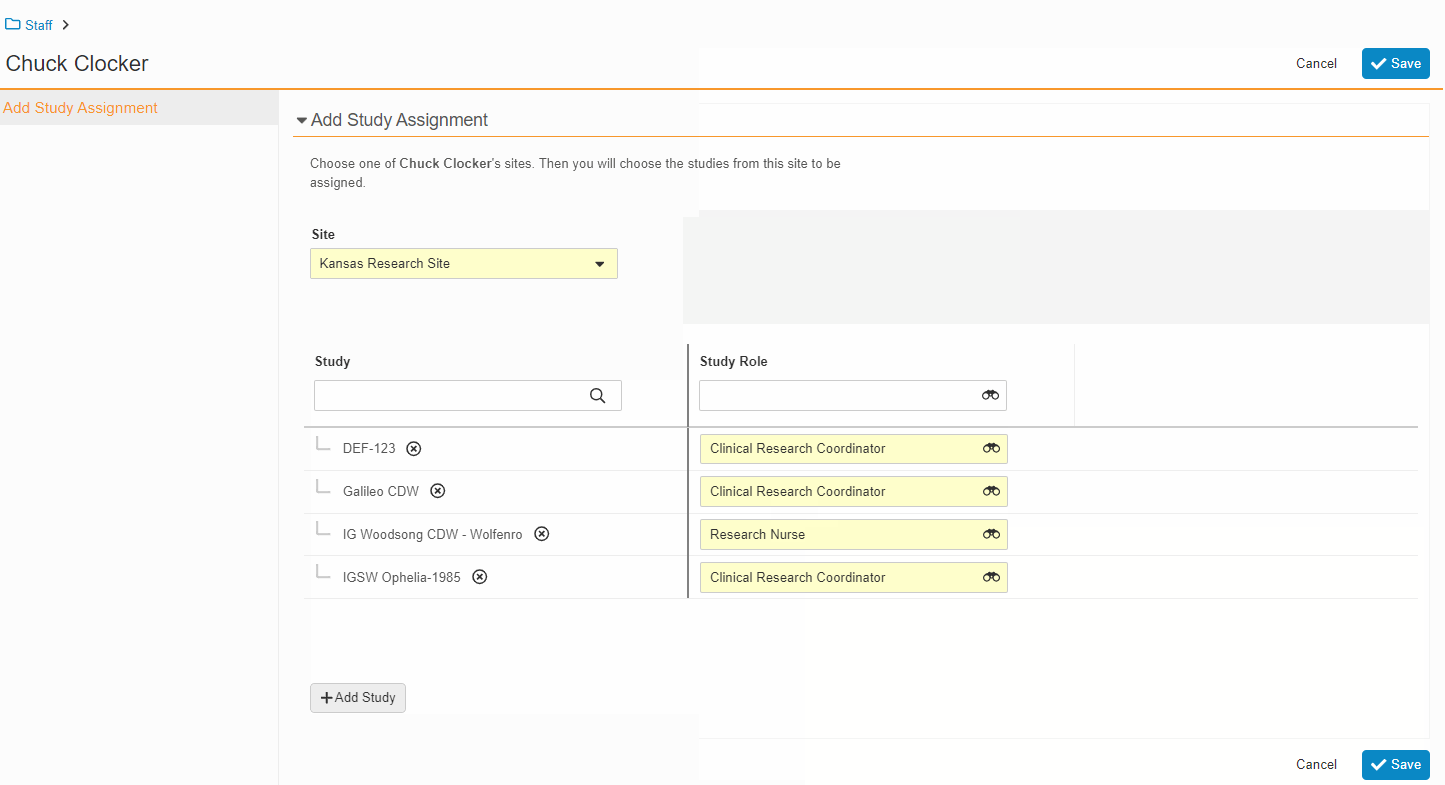
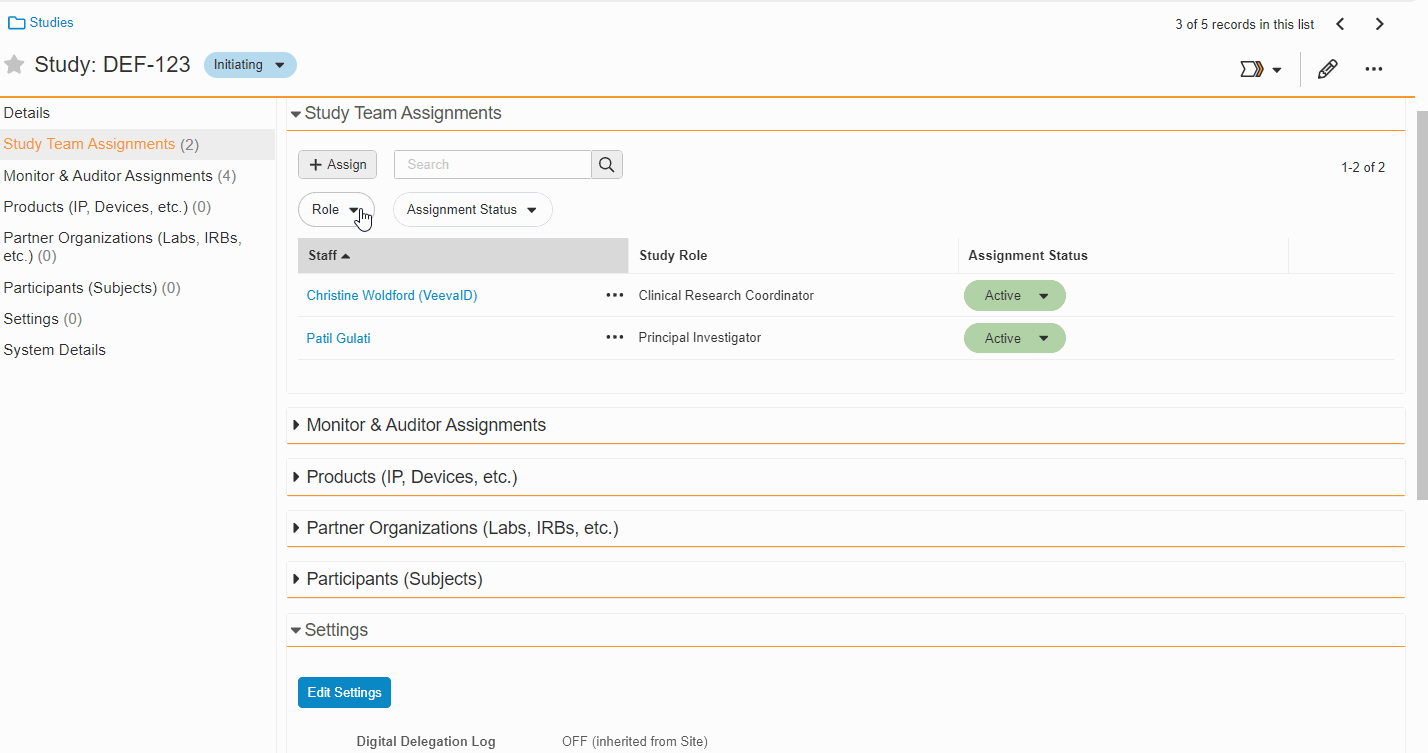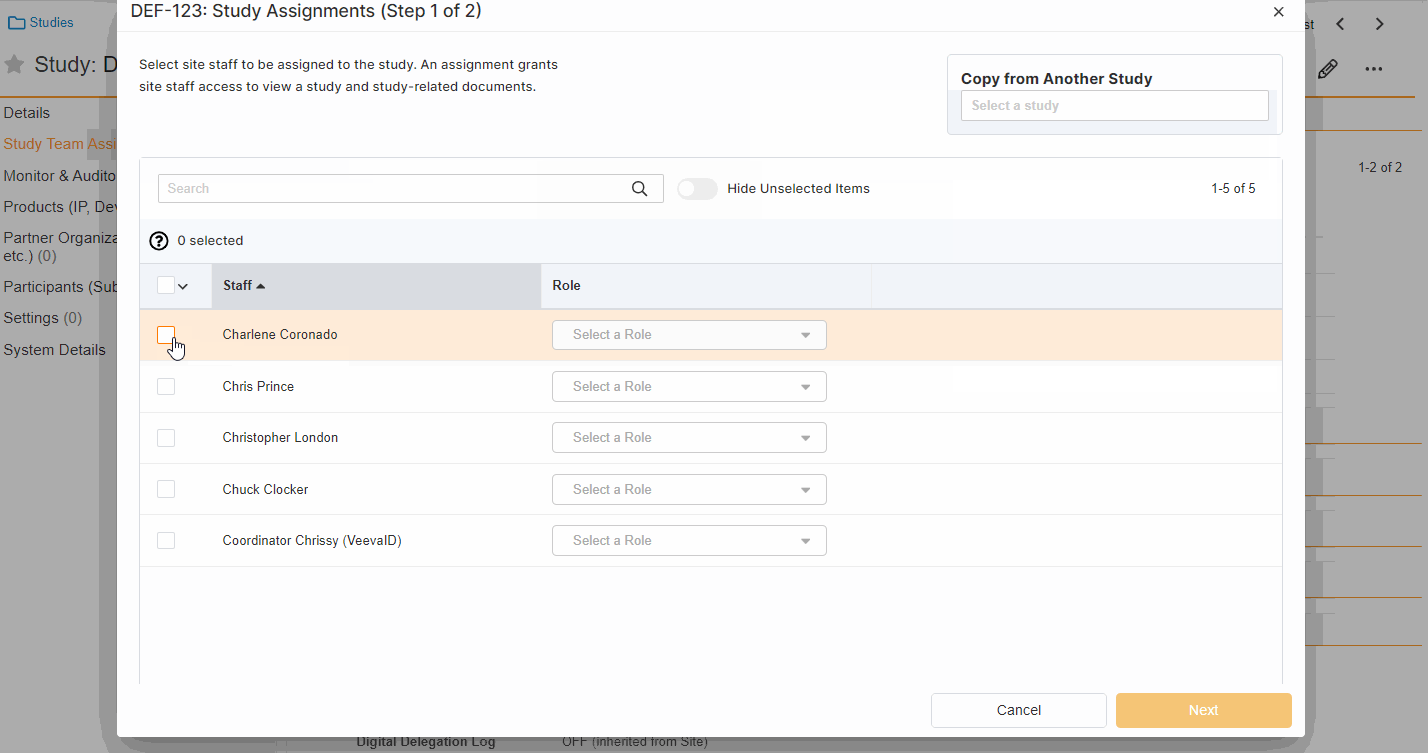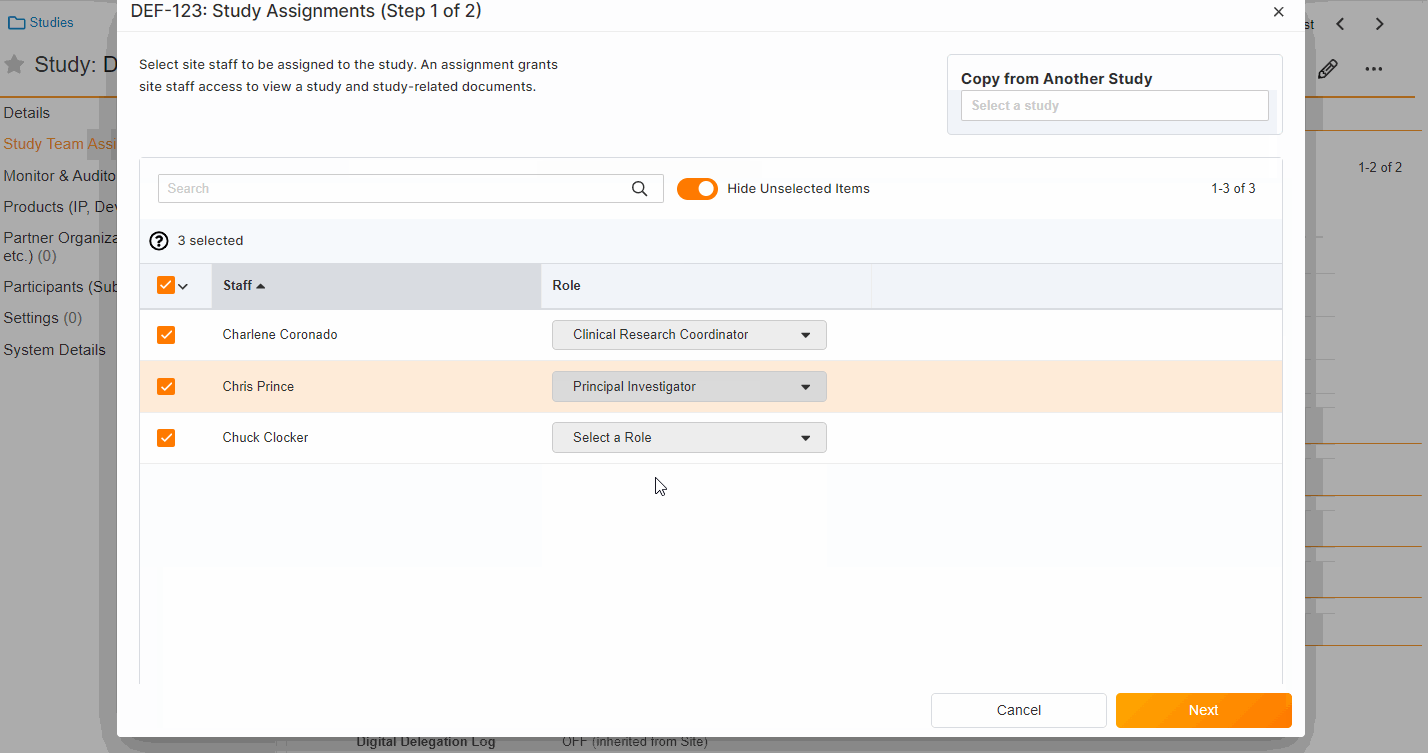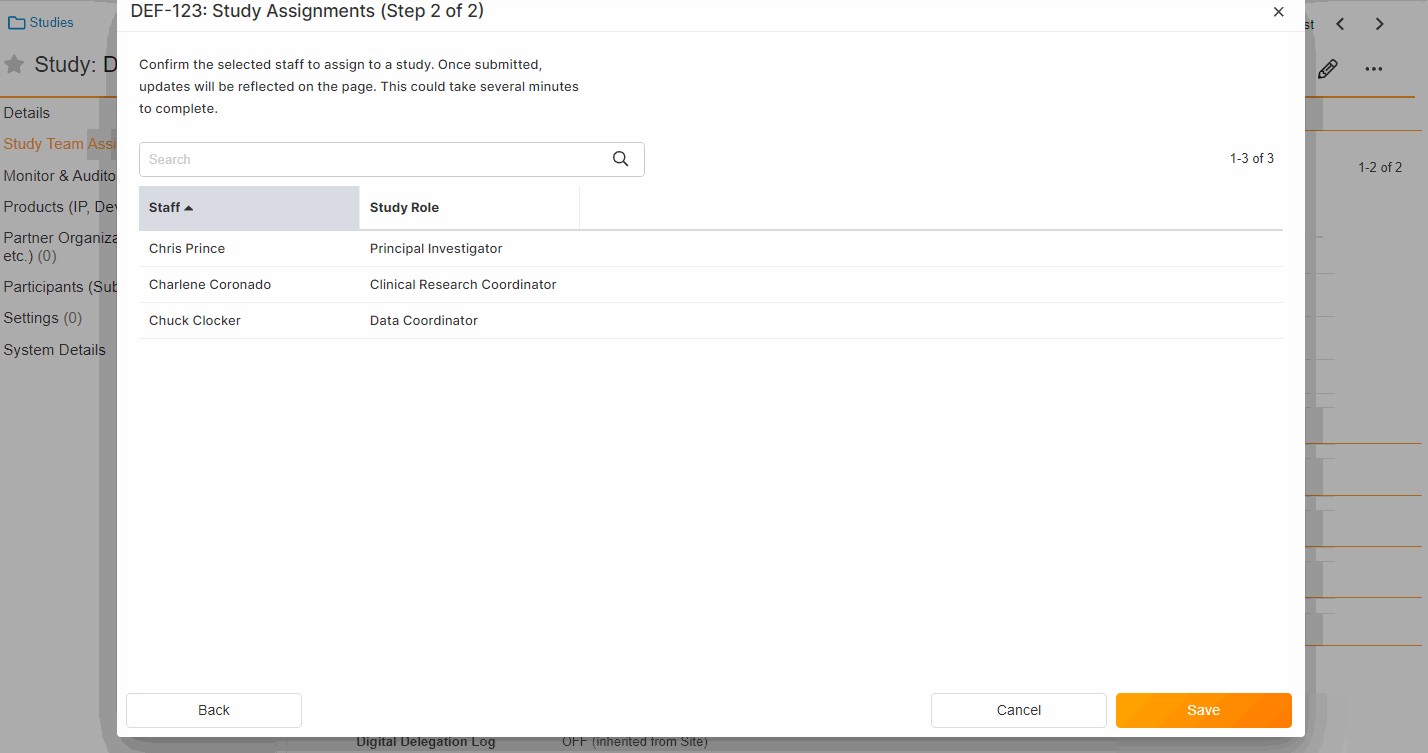
Study Team Assignments: Copy and Bulk Create
This feature enables Site Administrators to create study team assignments in bulk or to copy assignments from an existing study. The +Assign button prompts a dialogue to select one or multiple users to assign in a single process. The dialogue also includes the option to copy assignments from an existing study. This action populates the dialogue with the assignments of the existing study and then remains active for any needed adjustments. Additional enhancements to the Study > Study Team Assignments section include the ability to filter by Role or Assignment Status and to edit statuses directly in the Assignment Status column.Bulk add study to Staff from Administration > Staff > Study Assignments:
Bulk add Staff to study and copy study from Study > Study Team Assignments:
-
Edit Study Assignments on Staff Detail Page
This feature enables Site Administrators to edit the status of study assignments directly from the Staff Details Page in SiteVault eReg. This section of the Staff Details Page has also been updated with more filtering and navigation controls.For more information, see Study Team Assignments.
eConsent - eReg
-
Caregiver Support
With this eConsent/ePRO feature, patient caregivers are able to complete assigned surveys and sign consent forms.- ePRO: When enabling a participant for ePRO, site users can select an existing guardian, proxy, or caregiver for a patient or create a new one if none exist.
- eConsent (eReg & Study Connect): The Caregiver role is an option for Role selection fields when creating a signatory, sending an eConsent, or using the eConsent Editor. When consenting, caregivers can only sign signature blocks assigned to the Caregiver role; they cannot sign or answer questions in place of other signatories.
-
eConsent Custom Header and Footer Options
This eConsent Editor feature offers sites the ability to define their own custom header and footer text for print copies of their eConsents.
SiteVault Study Connect
eConsent - Study Connect
-
In-Person eConsent Enhancements in Study Connect
This feature provides the following navigational and usability enhancements to the in-person eConsent workflow in SiteVault Study Connect:- The name of the Signed Consent Forms section on a participant’s page is updated to Consent Forms.
- The View In-Person Code icon on the participant’s page is replaced by a Start Consent In Person button in the Consent Forms section.
- When you initiate the session, a new Start Consent In Person modal is available that enables you to start an in-person consent session using your current device or start a session on another device by scanning a QR code.
- Site-Specific Informed Consent Forms in Study Connect
With this feature, site-specific informed consent forms are accessible and can be sent to participants from Study Connect.
ePRO
-
ePRO Caregiver Support
With this eConsent/ePRO feature, patient caregivers are able to complete assigned surveys and sign consent forms.- ePRO: When enabling a participant for ePRO, site users can select an existing guardian, proxy, or caregiver for a patient or create a new one if none exist.
- eConsent: The Caregiver role is an option for Role selection fields when creating a signatory, sending an eConsent, or using the eConsent Editor. When consenting, caregivers can only sign signature blocks assigned to the Caregiver role; they cannot sign or answer questions in place of other signatories.
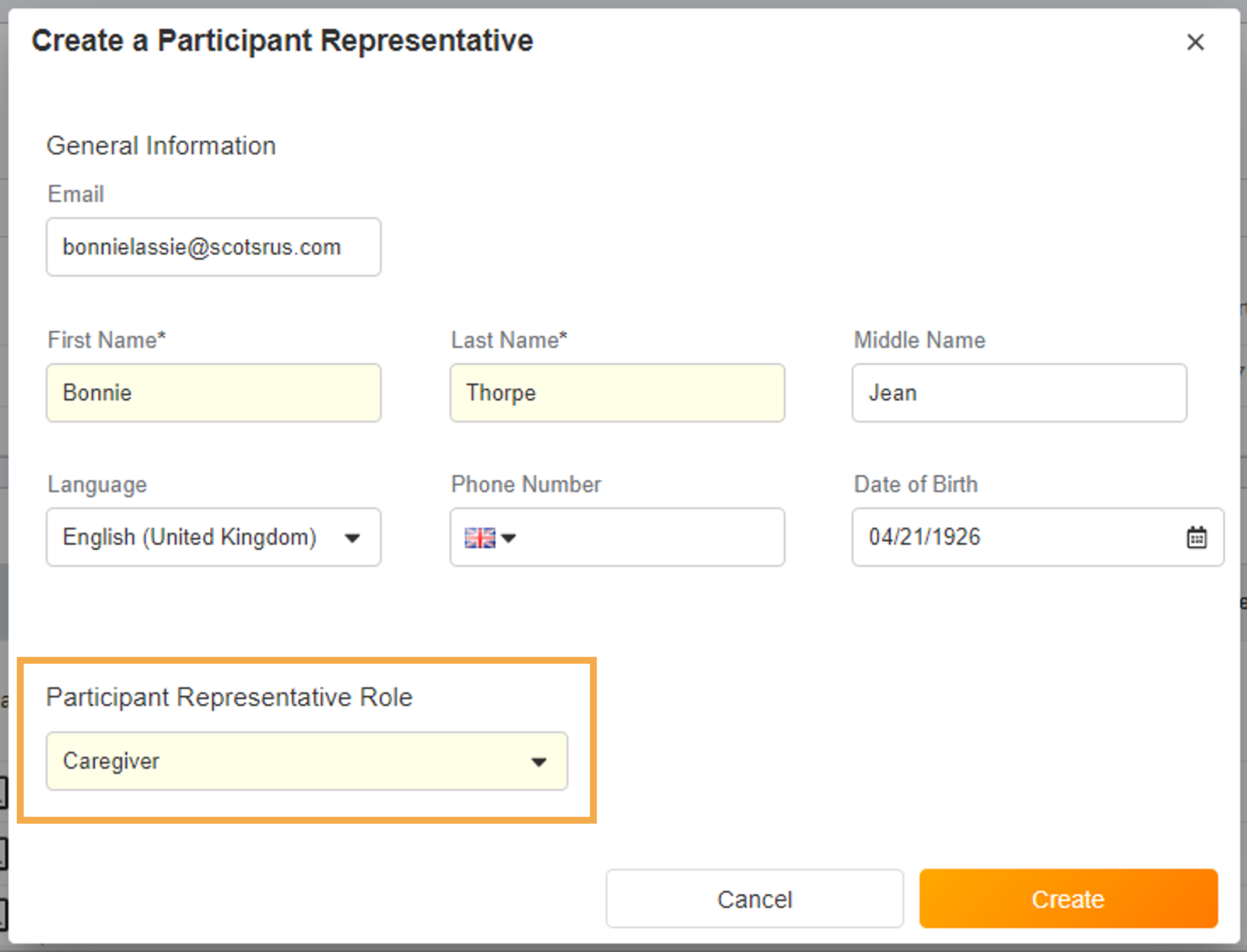
- Surveys Sent Based on Assigned Groups
This feature allows sponsors to define a list of groups for a study and assign those groups to specific schedules and events, and allows sites to define which group a participant belongs to. Related events and schedules are only created or triggered for a participant when they match the participant’s assigned groups.
-
Automatically Populate Actual Datetime with Protocol Expected Date
This feature allows sponsors to specify an anchor event and offset days when they create an event. When defined, this information automatically populates the datetime for related events. The site staff are responsible for updating the value with the actual datetime when it’s known so that the correct dates are calculated for the other events connected to the anchor event. -
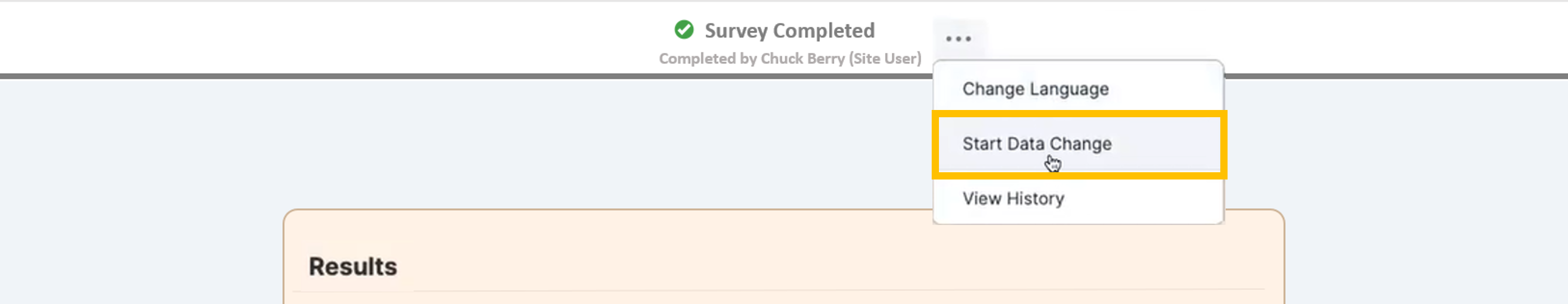
Controlled Edit of Survey Responses (Data Changes)
This feature allows site staff greater control over their source data, which enables them to process data changes without involving sponsors or vendors. The user is required to provide a reason for all their changes after the data is changed, and the reason is stored in the audit trail. To ensure compliance with regulations, the user can navigate back through the survey’s history to view all the changes. The sponsor now has access to a report that details all the changes that have occurred on their study.
Document Exchange
- New Exchangeable Document Types on Connected Studies
This feature adds the following document types to the list of document types that can be transferred to a Clinical Vault on a Connected Study:- Acceptance of Marketed Product Material
- Clinical Study Report
- Equipment Log
- IP & Supply Shipping
- Regulatory Authority Response
- Regulatory Submission.
Additional New Features or Enhancements
- Approve Person Profile Documents Received via Site Connect
This feature extends the Change Status to Current user action to documents on the Person Profile lifecycle, in the Received from Sponsor/CRO state. The action is present for Administrators and Site Staff with the Site Profiles permission.