SiteVault Release Highlights
SiteVault eReg
Study eBinder
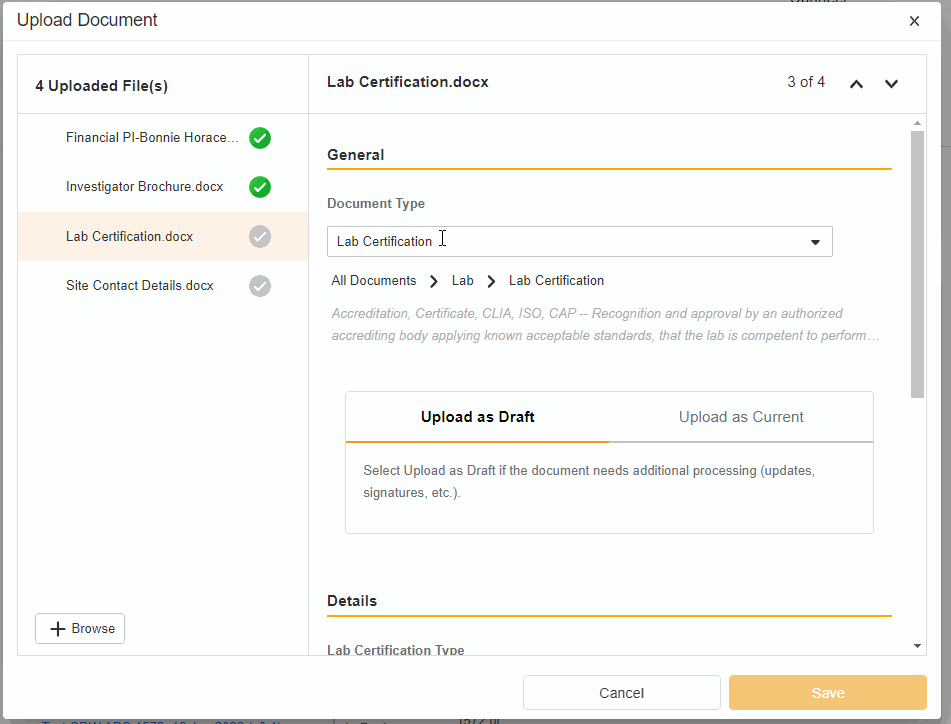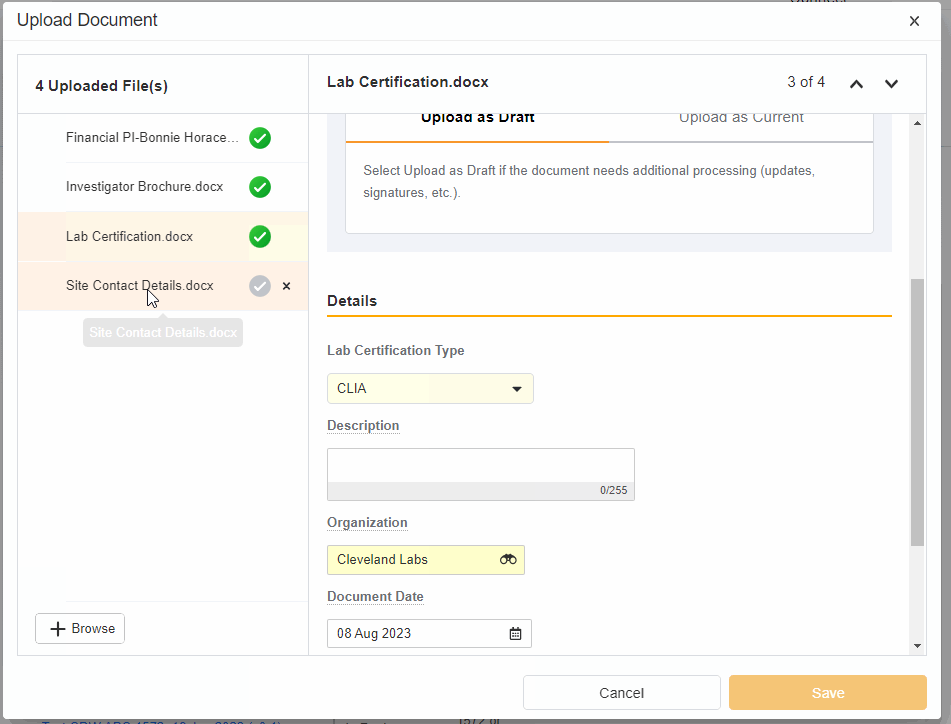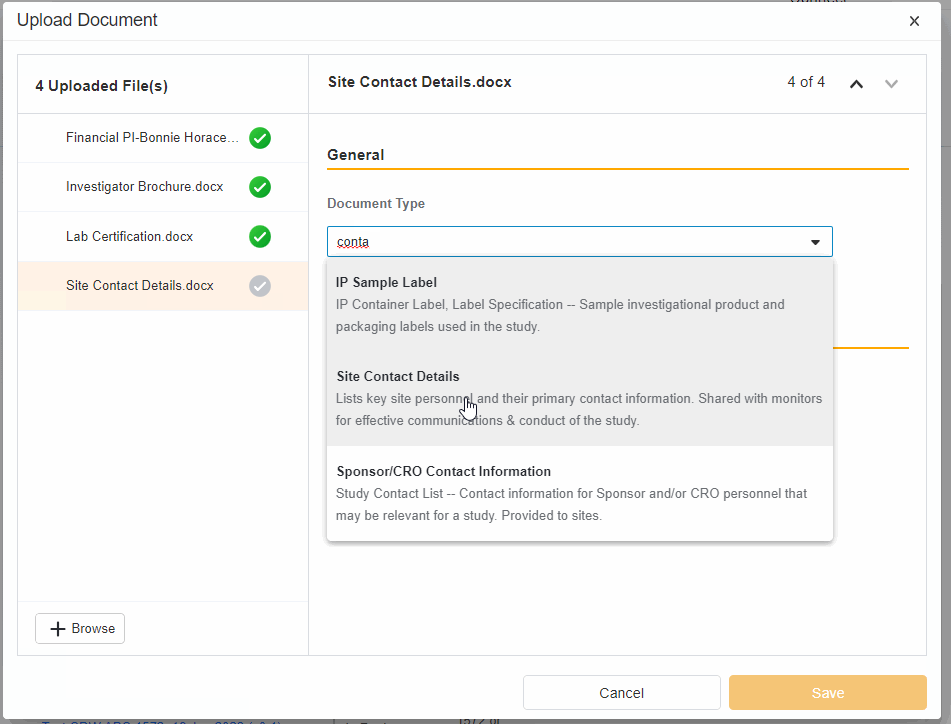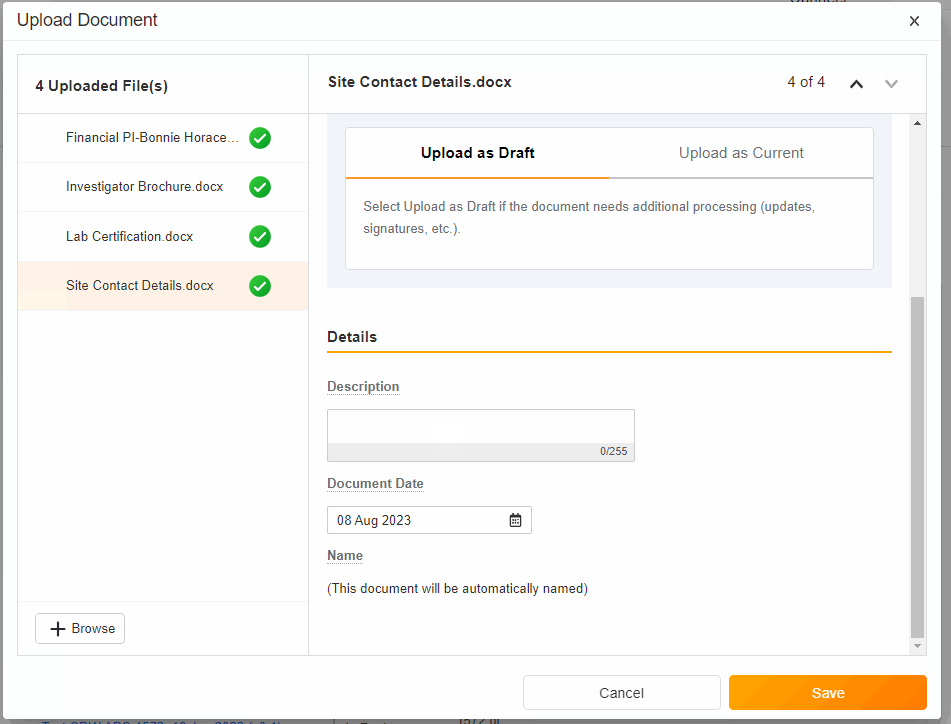
- Upload Multiples Files
This highly-requested feature expands the current eBinder upload functionality to include the ability to simultaneously upload multiple files with varying document types.
-
Bulk Source Upload
This feature shifts the bulk uploading of Source documents from the Source Upload tab to the Study eBinder. The helpful tools that allowed you to quickly populate fields in the Source Upload tab are also present in the eBinder Bulk Source Upload feature. Additionally, you have the ability to upload documents directly to their steady state. -
Create a Study, Organization, Product, or Participant from eBinder
This feature grants the ability to create a new Study, Organization, Product, or Participant directly from eBinder. Studies can be created from the Study Selector, while Organizations, Products, and Participants can be created during document upload.
Managing Users
- Bulk Assign Staff to Studies
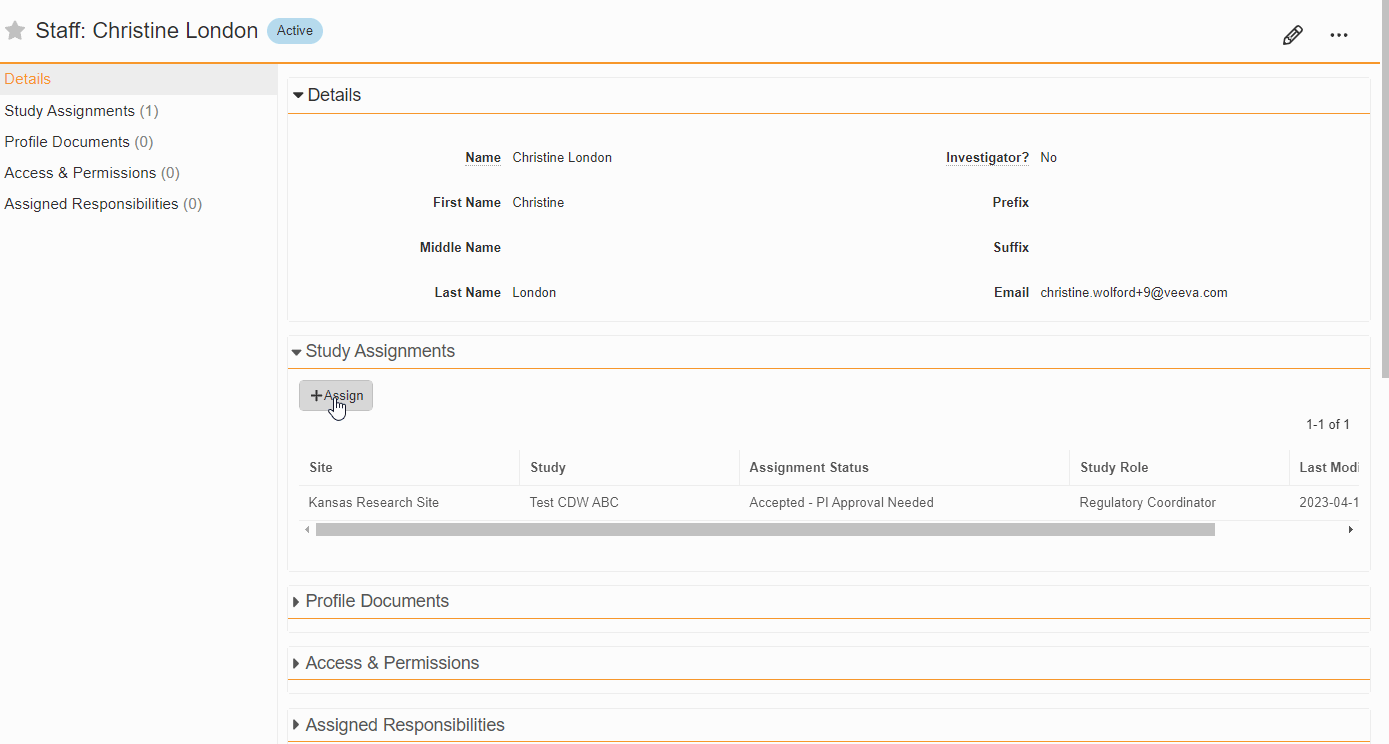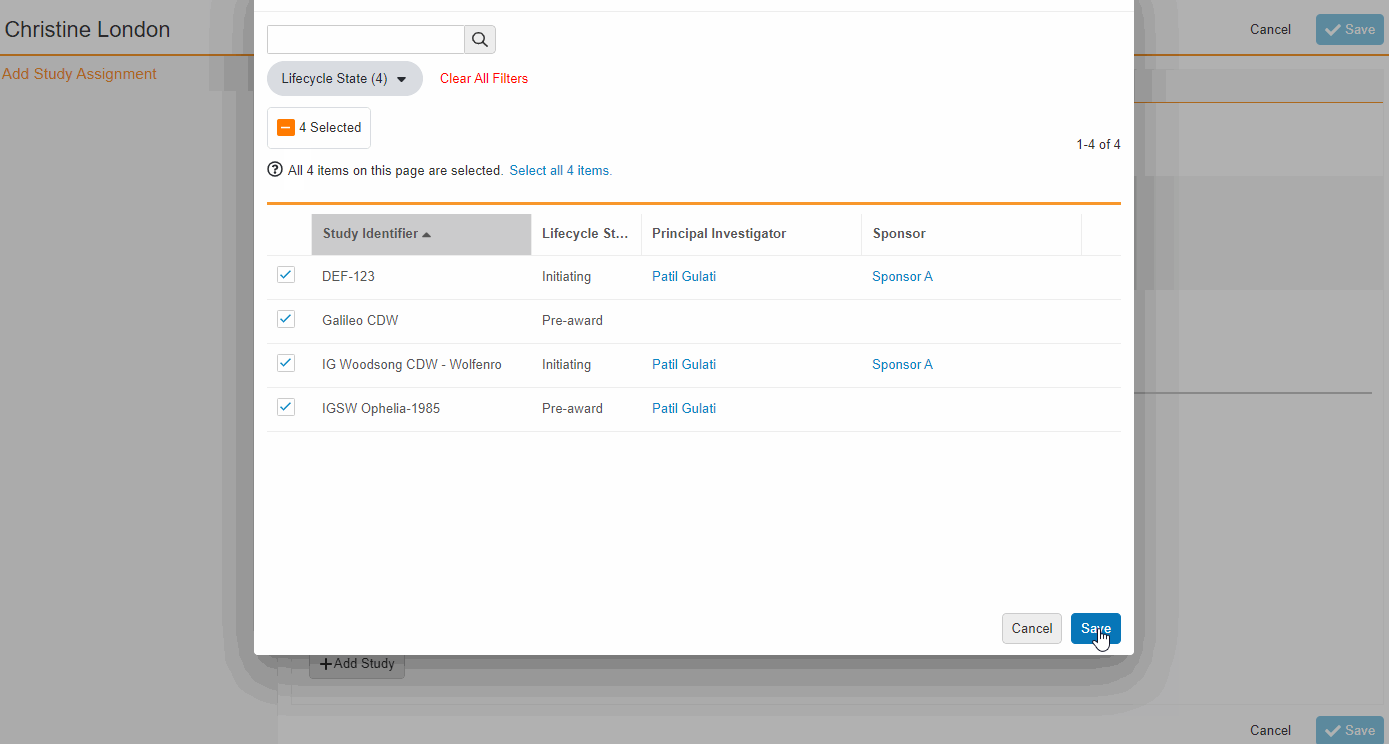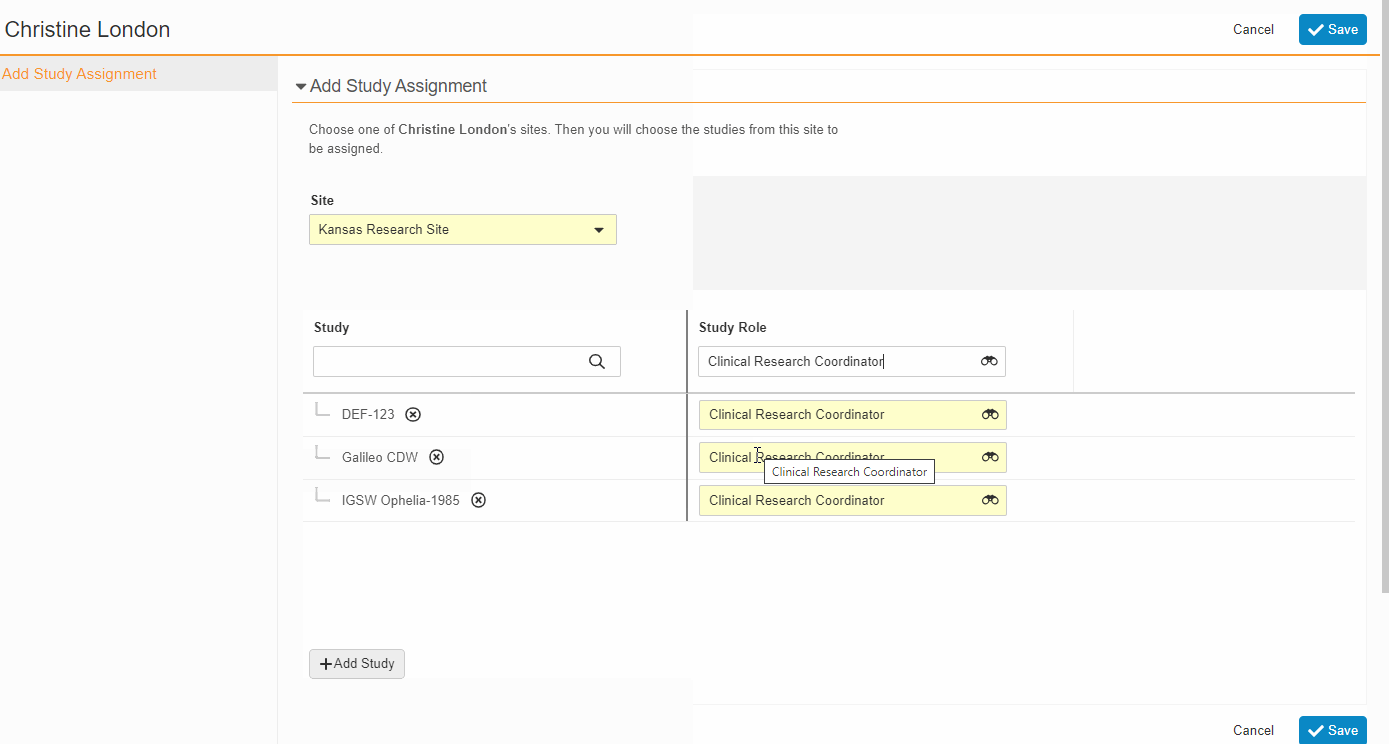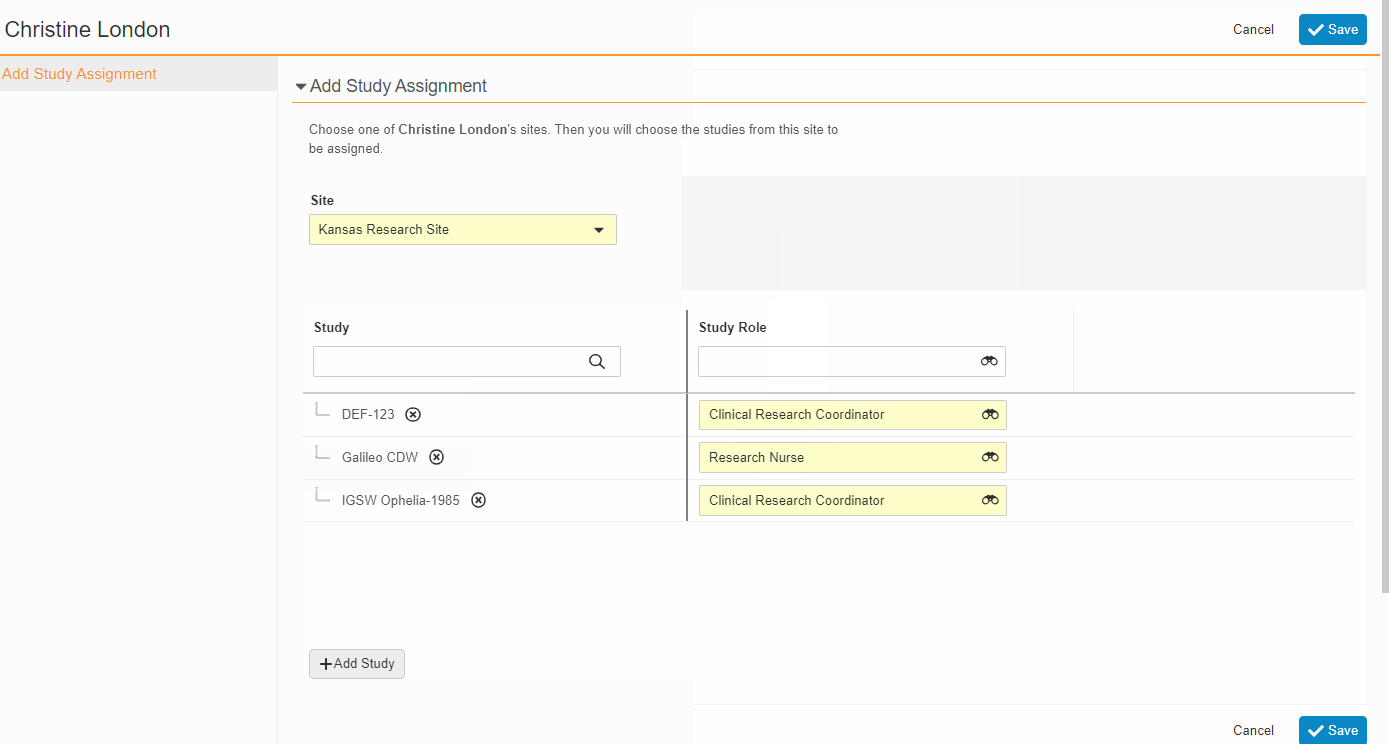
From the Staff Details Page (Administration > Staff), an Administrator can create Study Team Assignments for one or many studies. This enhancement includes the ability to apply a Study Role to all selected studies with a single click. The Staff Details Page contains a new section titled Study Assignments that displays assignments of all statuses, replacing the Active Study Assignments section.
-
Fully Inactivate a User and Their Study Assignments
This feature enables you to inactivate a user and their Study Assignments in a single step. This feature is available for managing both staff and external users. -
Staff Creation Updates
The Staff creation process is enhanced with an improved display of existing accounts utilizing the entered email address. -
Digital Delegation: Automatically Populate End Date when Inactivating Study Staff
For those using Digital Delegation, this feature expedites the process of inactivating a study staff member by adding their end date to all their delegations. When a Study Assignment state moves to Make Inactive, the user’s delegation End Date-Time fields inherit the assignment end date-time if they are empty or reflect an end date-time after the assignment end date-time. The delegation Start and End Date-Time fields remain editable while the assignment is in the Make Inactive state.
Managing Documents
- Site Documents
You can use SiteVault to manage site-specific, non-study documents such as standard operating procedures (SOP), work instructions, and policy memos. These documents are stored in the Document Library, visible to Staff users once approved. Site Admins can create the documents and use workflows to send them for review, eSignature, and site staff training.
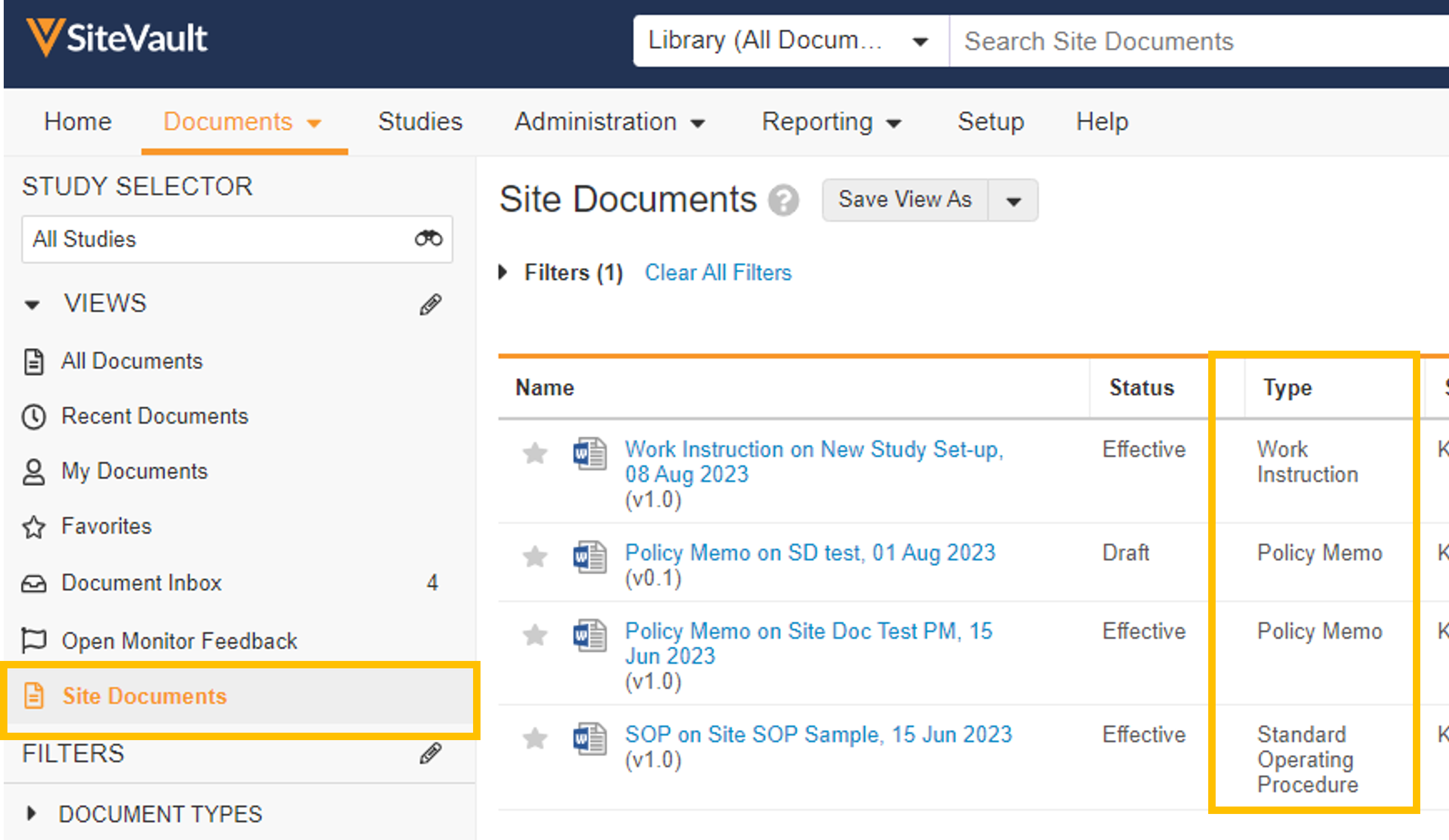
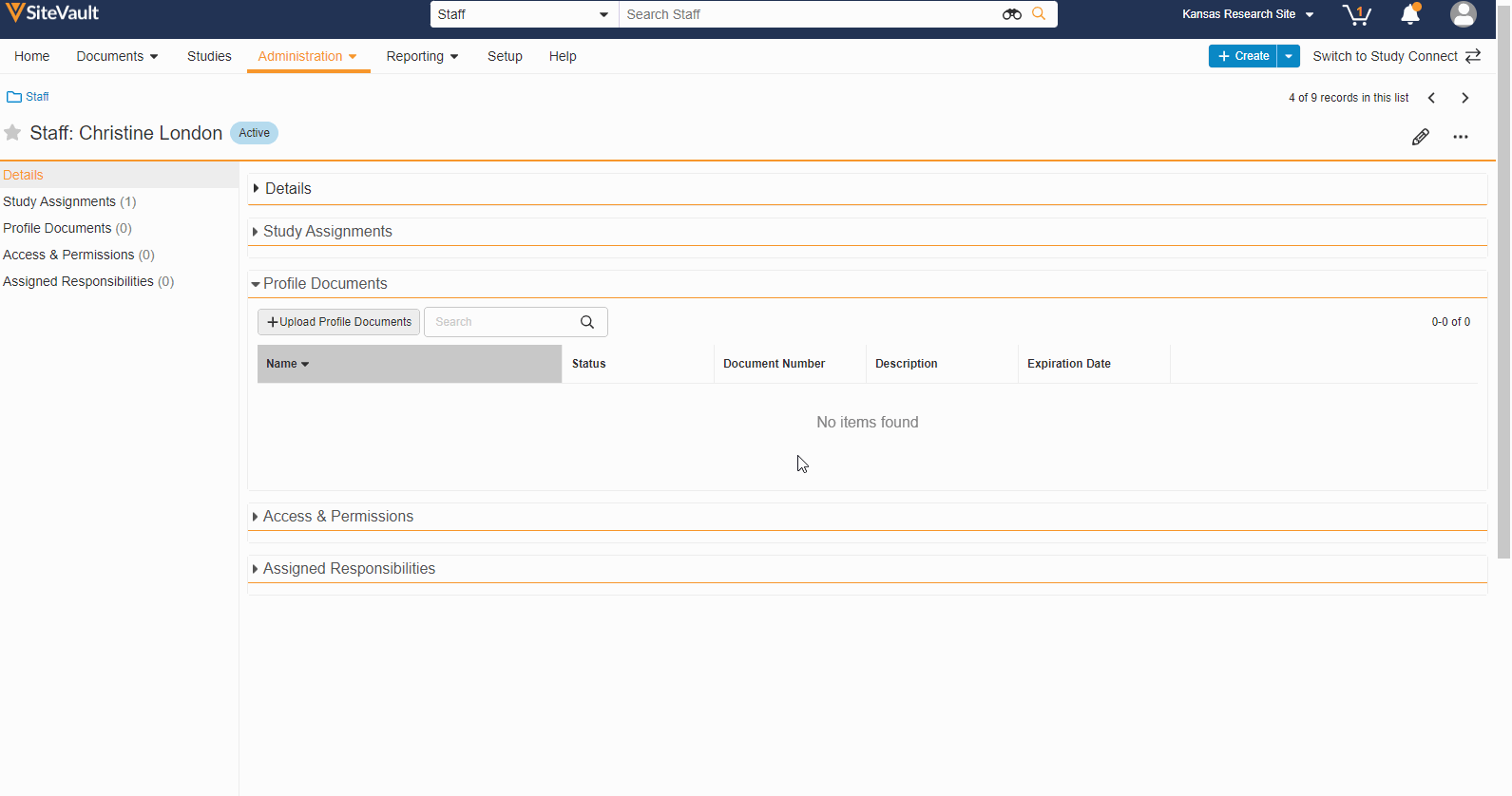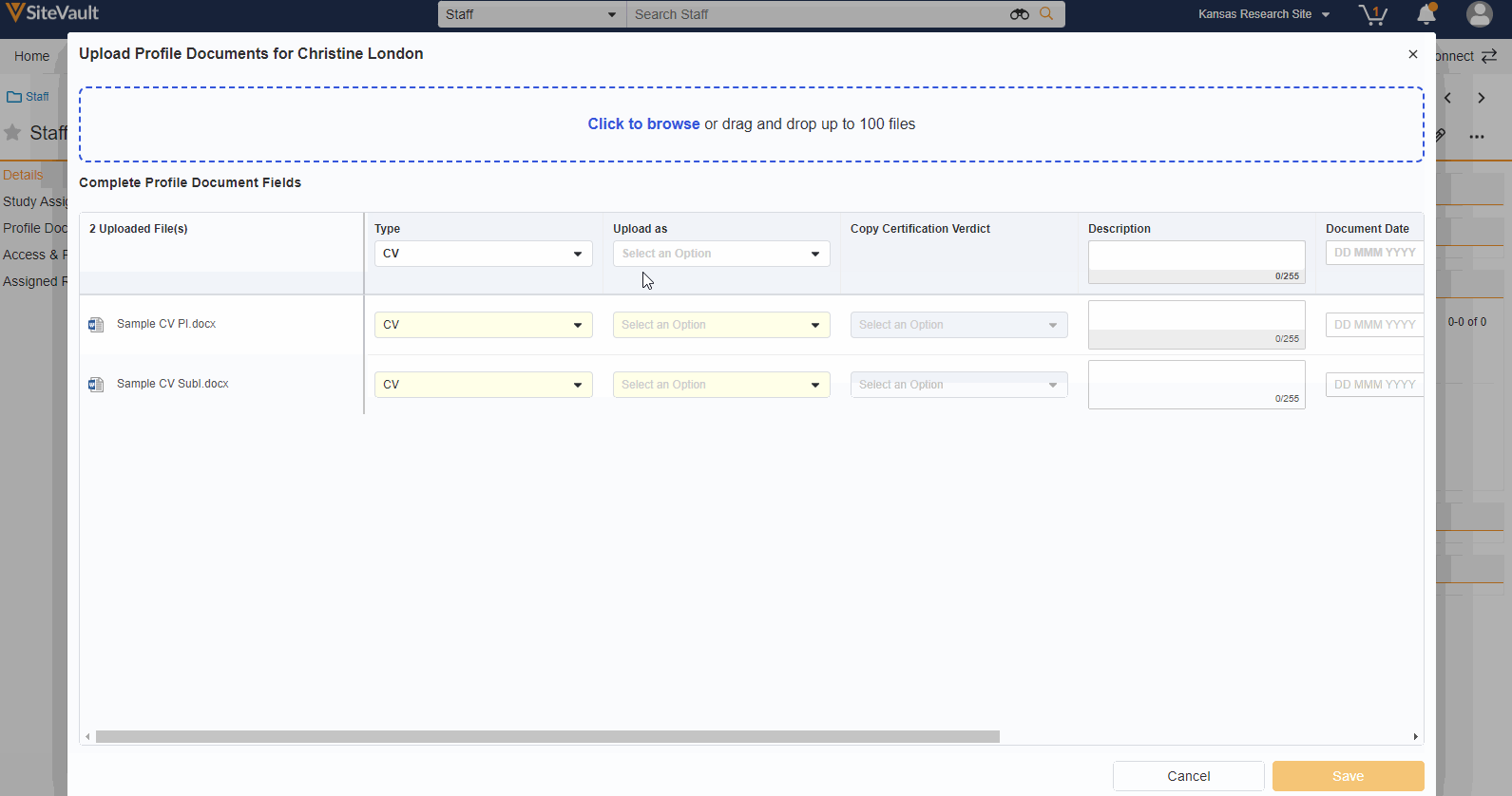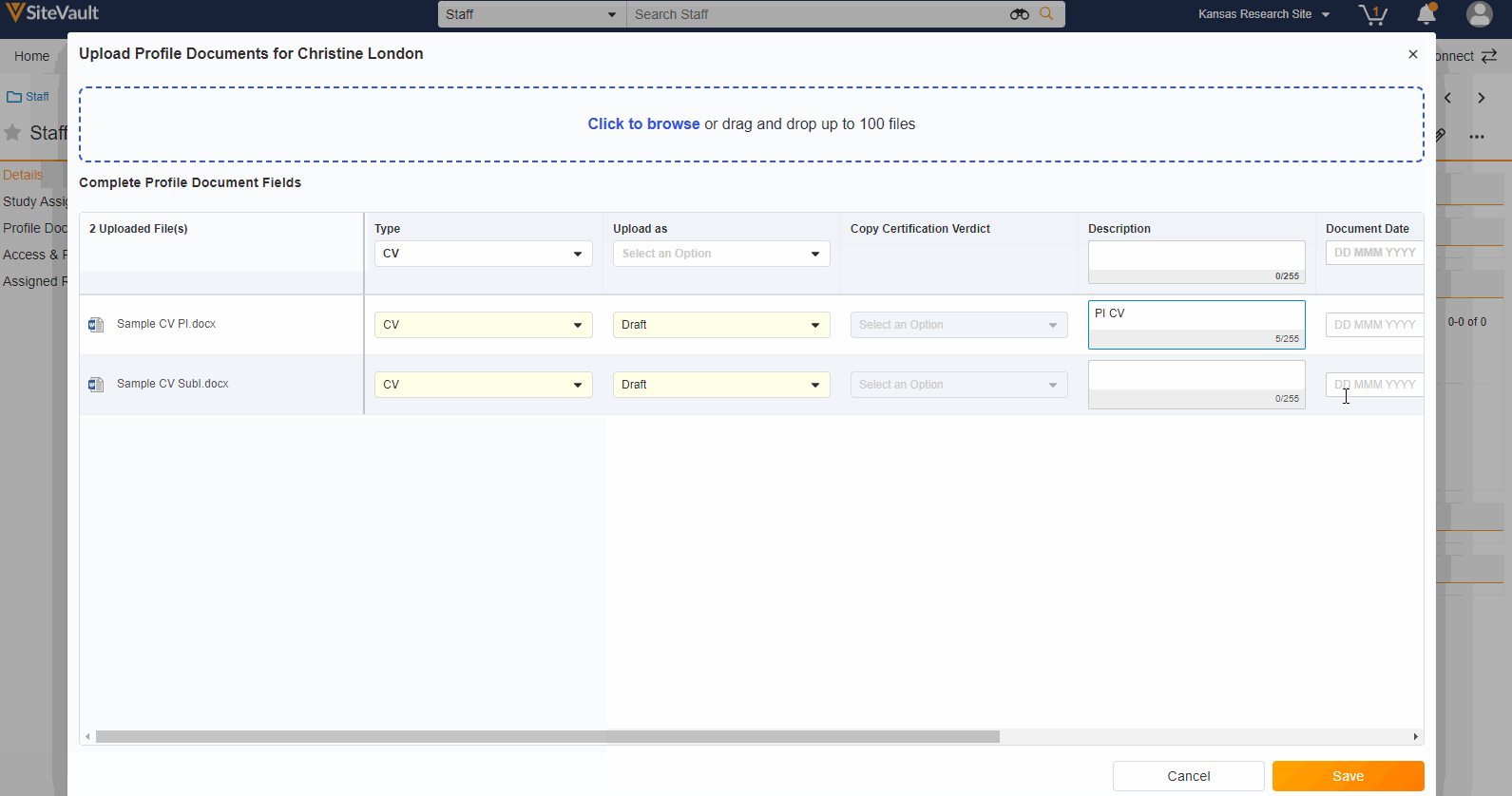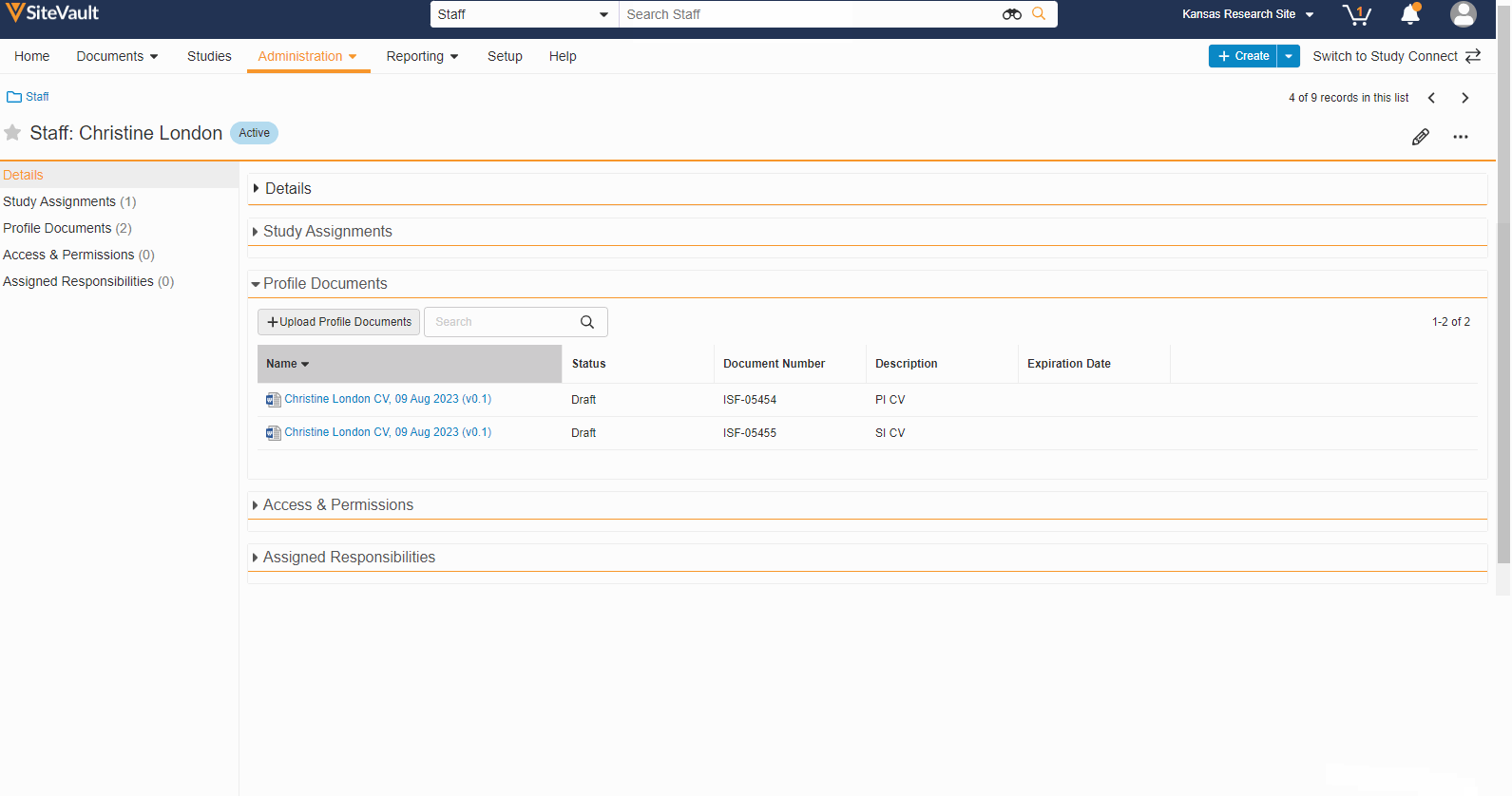
- Staff Profile Documents List
A Staff’s profile documents can be viewed and managed from the new Profile Documents section on the Staff Details page. From this section, Admins will also have the ability to upload documents (on the Person Profile lifecycle) directly to the Current state.
-
Workflow Instructions
When starting a document workflow, the workflow initiator can provide recipients with free-text instructions. These instructions will appear in the All and My Tasks notifications as well as on the document task ribbon.This feature is available on the following document workflows:
- Read and Understand
- Review Issues Found (Monitor)
- Send for eSignature
- Send for PI eSignature
- Send for Review
-
Update Document Reuse Logic
When activating a new Staff or Organization on a study, the most recent steady state version and any subsequent draft versions of profile documents are filed to the study. Previously, only the most recent version was filed to the study, regardless of document state. -
Participant ID Code List Document Type Update
The Participant ID Code List document type is now on the Draft to Current - Source document lifecycle. Files associated with this document type cannot be downloaded by external users.
Additional New Features or Enhancements
-
SiteVault Inviter
With this feature, Sponsors/CROs can assist sites with signing up for SiteVault, initiating the process from within Clinical Operations. Clinical Vault users can enter a site staff email address into the Inviter to identify if a site is already using SiteVault. If a site is using SiteVault, the USN is visible in the Clinical Vault. If a site is not using SiteVault, the site is invited to sign-up and the USN is available in the Clinical Vault upon sign-up completion. -
Patient Navigation Update
Staff users can continue to view, create, and edit Patient records by navigating through a study (Participant); however, the Administration > Patients tab will no longer be visible. -
Additional Languages with Locales
This feature adds the following four language locales to the list of available languages for document and patient records in SiteVault.- Czech Czechia (cs-CZ)
- Dutch Belgium (nl-BE)
- English Canada (en-CA)
- English Ireland (en-IE)
- French Belgium (fr-BE)
- French Canada (fr-CA)
- French Swiss (fr-CH)
- German Austria (de-AT)
- German Swiss (de-CH)
- Italian Swiss (it-CH)
- Spanish US (es-US)
For more information, see Supported Languages.
SiteVault Study Connect
ePRO
-
eClinRO in Study Connect
This feature expands the current ePRO functionality to include other types of Electronic Clinical Outcome Assessments (eCOA) and enhances the user experience when accessing, viewing, and interpreting surveys in Study Connect.Site users can:
- Manage and submit electronic clinician-reported outcome (eClinRO) surveys in Study Connect
- View completed ePRO and eClinRO surveys in SiteVault
- View eClinRO-related events in audit trail data exports
From a participant event in the participant record in SiteVault, site users can view a survey list related to that participant event and complete and submit the surveys. When a site user begins an eClinRO survey, it’s locked and made viewable in read-only format for other site users until the responding user submits, becomes inactive, or exits the survey. Site users can view completed eClinRO surveys, and audit trail data exports now include eClinRO-related events.
Studies using only eClinRO surveys are not required to approve the collection document prior to enabling ePRO for the study. When upversioned collections include both ePRO and eClinRO surveys but only modify the eClinROs, the collection version for participants is automatically updated.

-
eClinRO and ePRO - Participant Event Enhancements
This feature provides site users with an enhanced participant event list in Study Connect. They can drill into a participant event to view all related ePRO and eClinRO surveys. Each survey is identifiable by its status, and the user can begin new or view completed surveys. Additionally, users can toggle the list to view only the available surveys. When entering eClinRO surveys or viewing completed eClinRO or ePRO surveys, the site user can switch between the languages that the survey supports. -
Survey Compliance Warnings
This feature allows site users to view survey compliance warnings in Study Connect. When participants have important surveys coming due or have missed surveys in the last seven days, a new widget on the Surveys Overview page displays detailed information about compliance for those participants. From the widget, site users can access participant contact information and add notes. Compliance warnings for surveys that are coming due soon are derived from the survey’s “Survey Due” site notification configuration, which is defined by a study builder. -
In-Person ePRO Survey Completion
This feature provides participants with the option of performing ePRO activity while visiting a site, without requiring a MyVeeva for Patients account. You can start a participant’s in-person session from the participant event list in Study Connect. Participants can either complete their surveys on your site’s device or on a separate device. During an in-person survey session, MyVeeva users can complete any study surveys that are currently available to them.

-
eClinRO and ePRO - Data Export Enhancements
This feature enhances survey, adherence, and audit trail data exports for sponsor and site users by including new data related to eClinRO. New SITE STAFF values can populate in Completed By columns related to eClinRO surveys. New ePRO Survey and eClinRO Survey values can populate in Survey Type columns. These additional values may impact any external post-export data loads that users have set up for data analysis. -
My Veeva for Patients PIN Access
This feature allows ePRO enablement for participants who prefer or cannot provide an email address or phone number. You can generate a Device Registration Code in Study Connect and provide it to a study participant or representative. When the participant or representative enters the DRC in the Android or iOS app, they’re prompted to create a device-specific PIN to log in and receive their consent forms, surveys, visits, and study documents during the study.

Document Exchange
- eReg Document Number Displayed in Study Connect
The eReg Document Number is visible from all Document Exchange Document tabs and Study Connect documents. You will now also be able to search for eReg document numbers within Study Connect.
- Document Exchange Document Tabs: Filed eReg Number column
- Study Connect Documents: eReg Document Number document field located in the Sponsor/CRO Information section
- Sponsor Name on Document Exchange Notifications
This feature adds the name of the sponsor to the subject line of Document Exchange emails and to the notifications in the Study Connect notifications panel.
Additional New Features or Enhancements
- My Veeva for Patients PIN Access
From eConsent or ePRO Participant pages, you can generate a Device Registration Code in Study Connect and provide it to a study participant or representative when a phone number or email address is not available. When the participant or representative enters the DRC in the Android or iOS app, they’re prompted to create a device-specific PIN to log in and receive their consent forms, surveys, visits, and study documents during the study.

-
Additional Details in Study Connect Action Needed Notifications
Study Connect weekly action needed notifications now include task details and due dates so you can more easily identify what action is needed. -
MyVeeva Study Home: Provide Site Contact Information
This feature enables sites to designate a primary contact that can be shared with participants in the MyVeeva for Patients application.

- Search Studies in Study Connect
With this feature, you can now search for studies in the Studies tab.

SiteVault Data Model Changes
With every release, we update the SiteVault data model to better support evolving needs and new feature functionality. See 23R2 Data Model Changes for more information.