Export All Study Documents
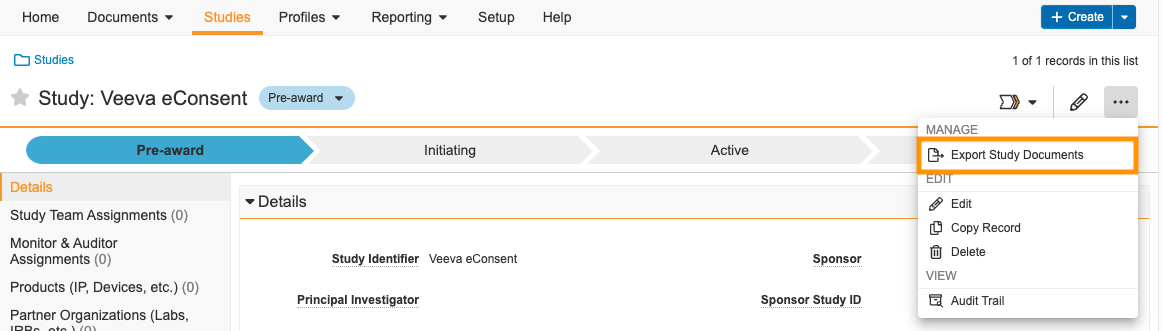
With this feature, Site Administrator users can export all major versions of all documents for a study. To do so, select Export Study Documents from the All Actions menu () on the Study record. Viewable renditions of the documents are exported as a .ZIP file and, when unzipped, are in the eBinder folder structure. The export also includes document audit trails (as .PDFs) and document metadata (in spreadsheet format). This enables you to provide a printed or exported version of your eBinder to your inspector, if requested.
See the Exporting Study Documents page for more information.
Updates to User Labels
In certain places throughout SiteVault, the label for Users has been updated to either User Profile or User Account. This is in preparation for a future feature where you can create a profile for a site staff member in SiteVault without creating an account.
Connected Studies
View Connected Study-Related Details on Documents

The feature makes it even easier for you to view and export tracking information on Connected Studies-related documents. For example, you can view whether a document version has been sent to the sponsor or CRO, the date it was initially sent, and the last sent date directly in a document’s metadata. In addition, you can select Connected Study Details from the document’s All Actions menu () to view more information regarding the exchange history, for example, the received or rescinded date and the full document comment history.
See the Viewing Document Exchange and Comment History page for more information.
New Exchangeable Document Type
This feature adds IP Excursions to the list of available document types that can be exchanged with a sponsor or CRO on a Connected Study.
Veeva eConsent
Simple Signing
This feature allows participants and other signatories to sign and submit consent forms in person without registering a MyVeeva for Patients user account. Being able to sign without an account enables signatories in demographics without email addresses or mobile phones to use Veeva eConsent within compliance regulations, increasing the scope of studies and participants that Veeva eConsent supports.
In SiteVault, you can now send eConsent forms without contact information. When a MyVeeva user is unregistered, the system requires the MyVeeva user to sign during an in-person consenting session and to draw their signature in place of dual-factor authentication.
See the Start the Consent Process page for more information.
Additional Signatories Enhancements
This feature includes general enhancements to the Veeva eConsent Additional Signatories feature, including making it even easier to create and manage Signatory records and providing users more flexibility in sending eConsent forms to signatories.
When site staff send eConsent forms to a participant who is associated with a proxy, the site staff member can choose to send the form to the participant instead of the proxy.
See Start the Consent Process for more information.
Patient Contact Information
With this feature, when a patient or signatory on a study updates their contact information in MyVeeva for Patients, their contact information is also updated in SiteVault. Site Administrator users receive a notification that alerts them to the changes.