Release Considerations Veeva eConsent is now generally available in SiteVault and Clinical Operations.
About the Release
Important Dates
The following dates apply to this MyVeeva for Patients release:
| Date | Event | Description |
|---|---|---|
| November 3 | Pre-Release | The 21R3 release is deployed to the MyVeeva for Patients pre-release environment and available for customers to explore from pre-release vaults. |
| December 3 | General Release | The 21R3 release is deployed and generally available. The MyVeeva for Patients 21R3 validation binder will be available in VeevaDocs to SiteVault Enterprise and Clinical Operations customers at this time. |
| To Be Determined | SiteVault Configuration | After the SiteVault change request and validation process, the latest Veeva eConsent functionality is available to sites who use SiteVault. See the SiteVault Release Notes and Validation Documents for more information. |
Release Information and Impacts
See the following resources for information about the impact of this release:
| Resource | Description | Updated |
|---|---|---|
| MyVeeva for Patients 21R3 Release Impact Assessment |
The release impact assessment analyzes the regulatory, implementation, and user impacts of the MyVeeva for Patients and Veeva eConsent features in this release. |
November 5 |
| What's New |
The What's New in 21R3 information below provides detailed explanations of the new features for MyVeeva for Patients and Veeva eConsent. |
December 3 |
| Digital Trials Platform Release Notes |
See the following release notes for more information about new features across the Digital Trials Platform:
|
See links |
What’s New in 21R3
Veeva eConsent
Additional Signatories
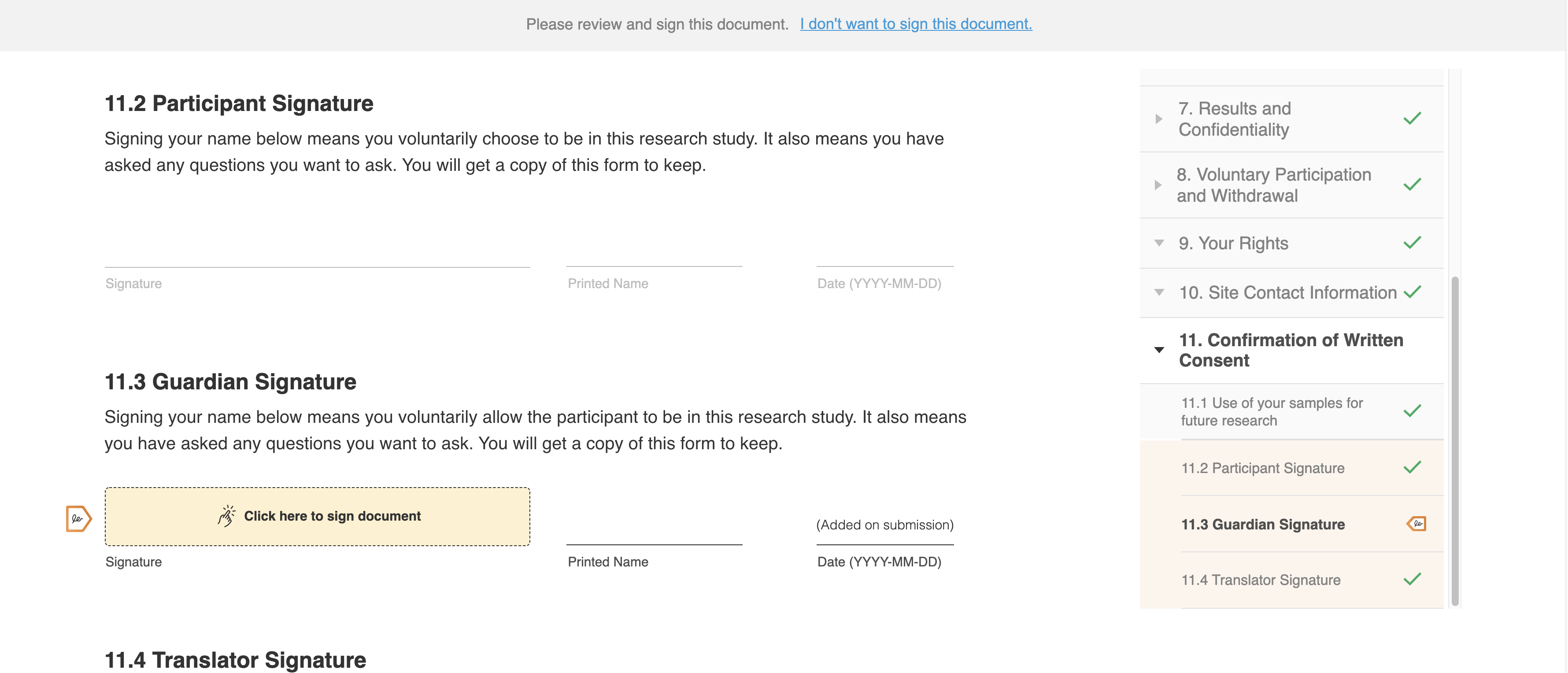
This feature allows sites and sponsors to capture additional signatures on eConsent forms for proxies, guardians, legally authorized representatives, witnesses, and translators. This enables sites and sponsors to use Veeva eConsent for additional studies and participants, such as guardians of minor participants or translators for foreign language speakers. The signatures are captured electronically and securely in MyVeeva for Patients.
See the following help topics for more information:
- Working With Sections and Content Blocks in Clinical Operations Help
- Consenting Participants in the Sitevault help
- Consent Forms in the MyVeeva for Patients help
Download and Upload Word Files for IRB Review
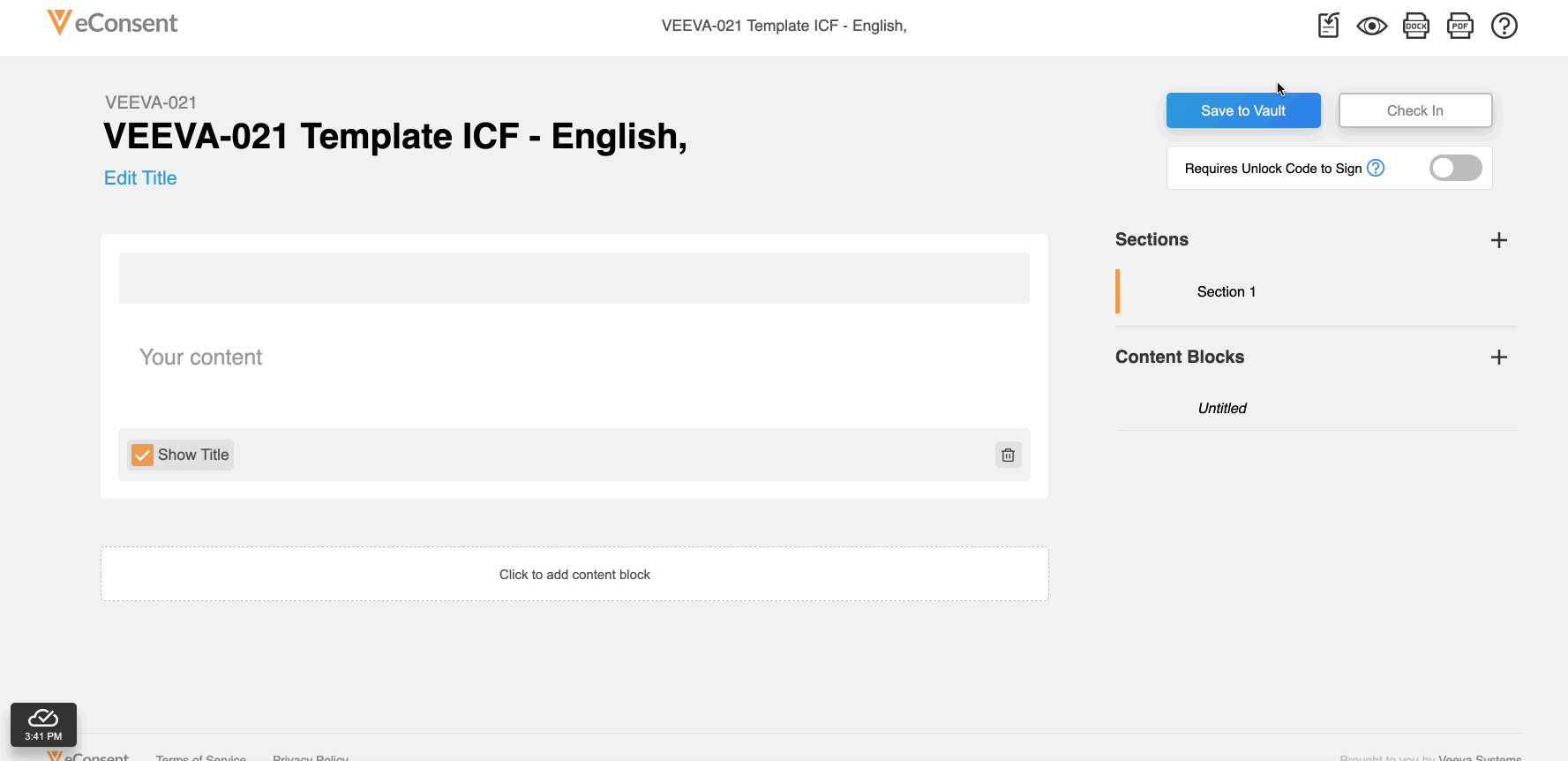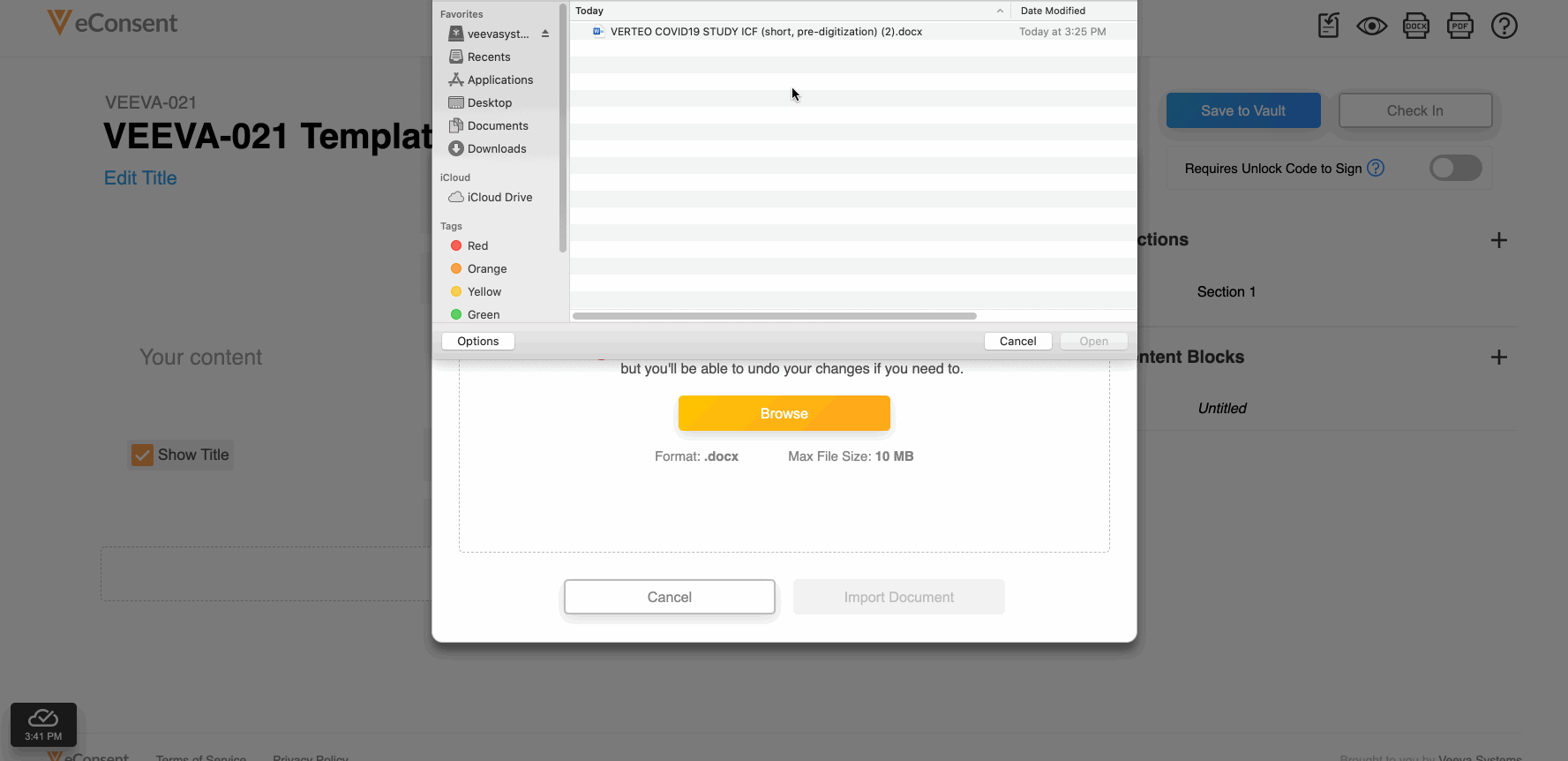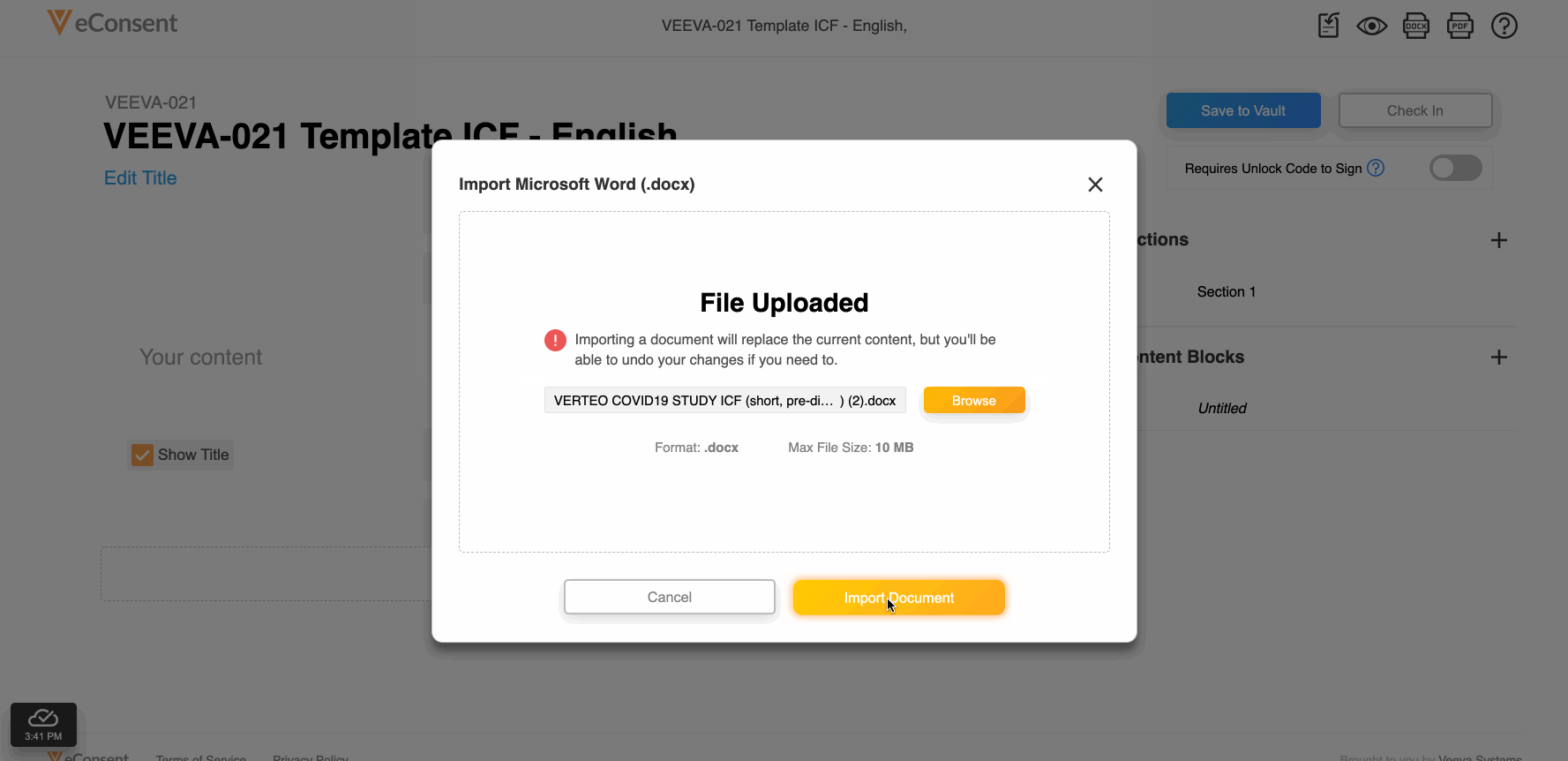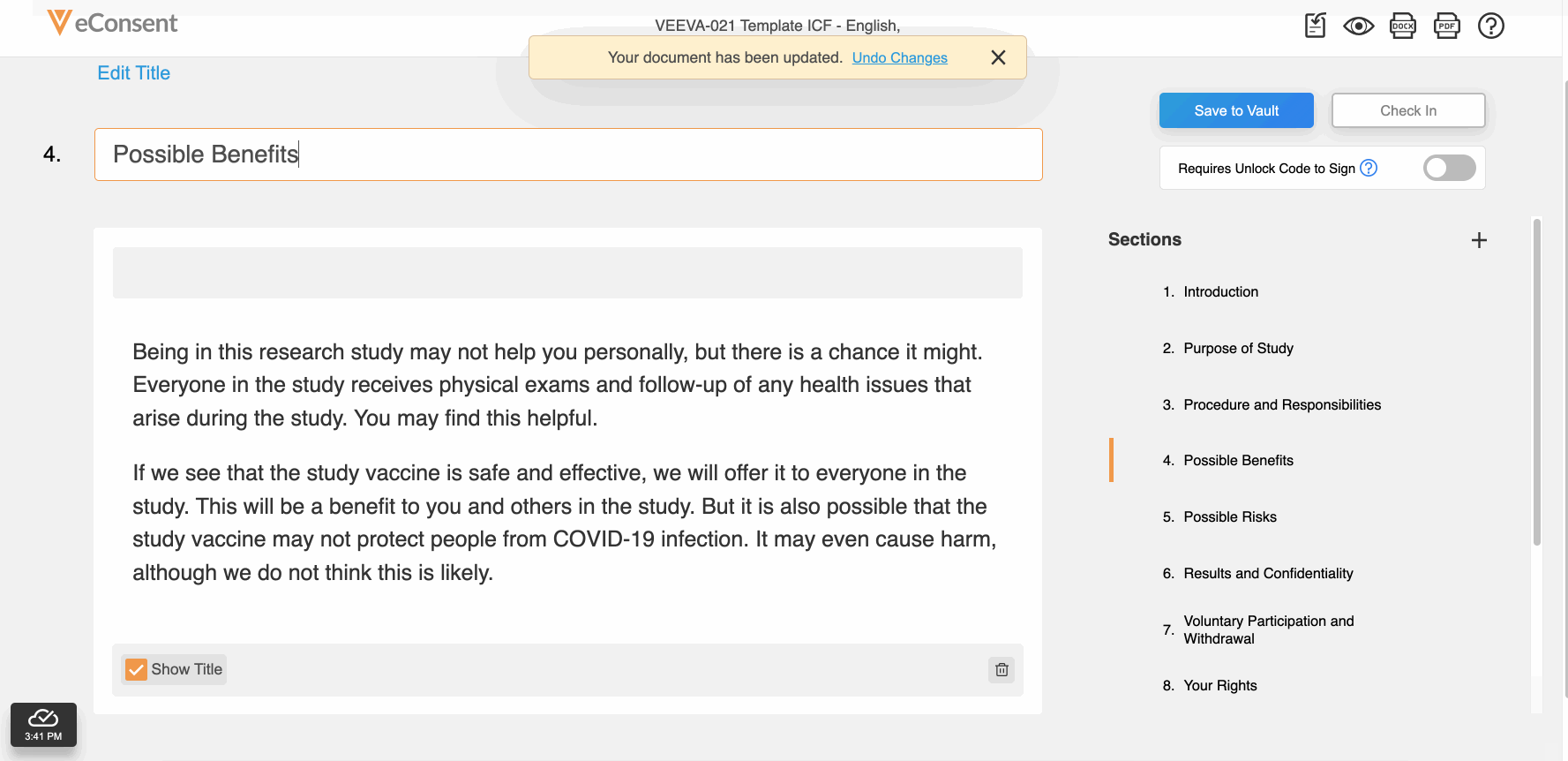
This feature allows site and sponsor staff to download and upload Microsoft Word (.DOCX) files in the Veeva eConsent editor. Staff can upload a correctly formatted .DOCX file, and the system automatically digitizes it.
This feature also facilitates easier document reviews with external parties such as institutional review boards (IRBs) by allowing digital eConsent forms to be exported as .DOCX files from the eConsent preview, modified, and uploaded back into the eConsent editor as a new version.
See the following topics in Clinical Operations Help for more information:
Unlock eConsent With Code (Review Without Signing)

This feature enables site staff to require MyVeeva for Patients users to have a site-provided unlock code to sign an eConsent form. Site staff can create the unlock code in the preview opened from SiteVault, and MyVeeva for Patients users can view the form but can’t sign it until they have the unlock code.
This allows the user to review the form before an in-person or virtual consenting visit while also ensuring that they have an opportunity to discuss the form with study personnel before signing.
See the following help topics for more information:
- Requiring an Unlock Code to Sign in Clinical Operations Help
- Consenting Participants in the Sitevault help
- Signing and Submitting Consent Forms in the MyVeeva for Patients help
Mobile
iOS App
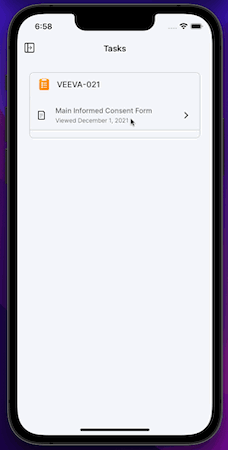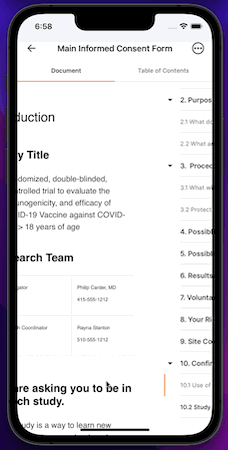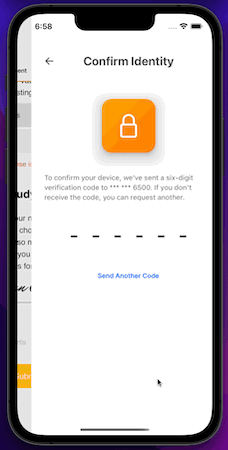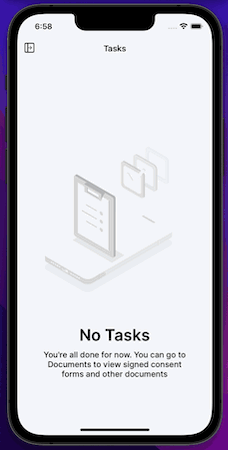
MyVeeva for Patients is now available as a mobile app on iOS devices. Participants and other users can use the app to view and download non-eConsent documents and to sign, decline, and download eConsent forms in an all-new, native mobile experience.
This release includes the features currently available in the MyVeeva for Patients web application and the 21R3 features that impact MyVeeva for Patients users.
See the MyVeeva for Patients Help website for more information.
Release Considerations Currently, users can only view profile settings in the iOS app. They can access the web application from the mobile app to update their phone number and email address.
Platform
Document Library (Share Non-eConsent Documents)
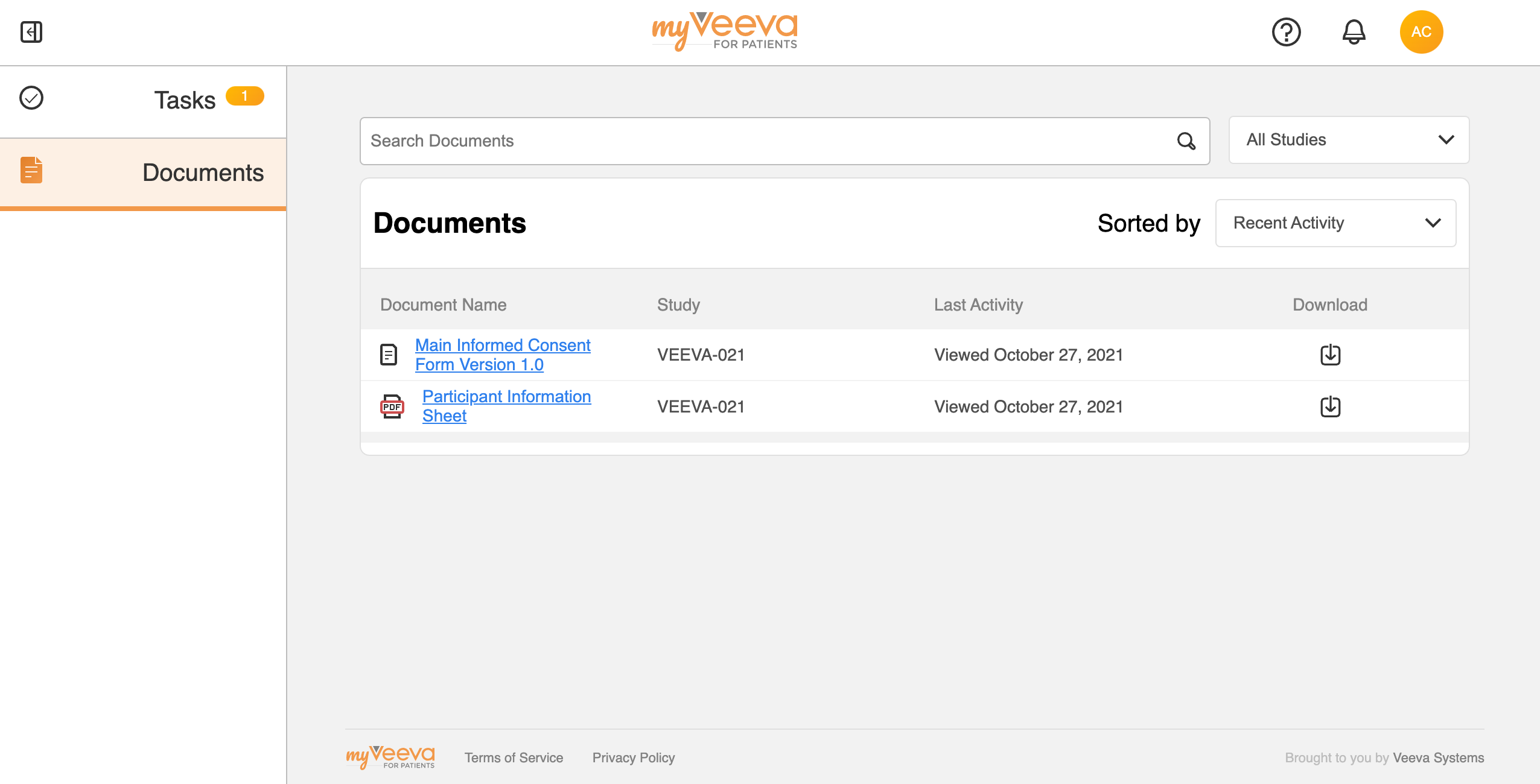
This feature allows sites to share non-eConsent documents with MyVeeva for Patients users before, during, or after a study. For example, sites can share product labeling, instructions for using home medical devices, collateral around payment methods, directions to the study site, or post-market product labeling updates.
Site staff can share such documents with new and existing participants, proxies, guardians, and representatives; and users can view and download the documents as PDF files in MyVeeva for Patients. Site staff can also retract the documents as needed.
UI Refresh (Navigation and Notifications)

With this feature, the MyVeeva for Patients UI is redesigned to enhance its visual appeal and accessibility, improve support for eConsent, and prepare for future features and product areas. Users can navigate between the Tasks and Documents tabs freely. They can also access their profile settings, switch between roles, view notifications, and search and filter documents. Additionally, the in-person consent flow is updated to support consent forms with multiple signatories.
See the MyVeeva for Patients Help website for more information.
User Profile Settings
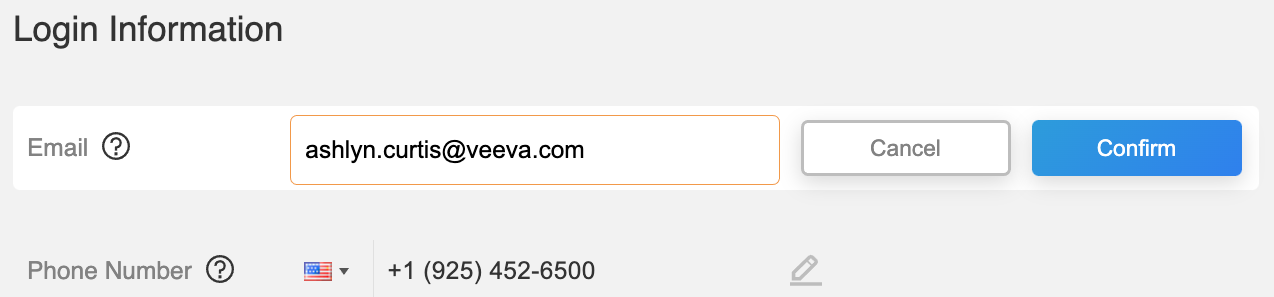
This feature allows registered MyVeeva for Patients users to securely update their email address and phone number in the MyVeeva for Patients web application. After the user verifies their identity, emails or text messages are sent to the updated address or phone number.
See the Account Settings page in the MyVeeva for Patients help for more information.
User Language Support for German, French, Italian, Polish, and Spanish

This feature enables MyVeeva for Patients users to view application text, emails, text messages, and the terms of use and privacy policy in French, German, Italian, and Polish. Additionally, the Spanish translations in the applications are improved.
See the Supported Languages topic in the MyVeeva for Patients help for more information.
Asia-Pacific Regional Instance Support
This feature enables sites, sponsors, and MyVeeva for Patients users to use MyVeeva for Patients in an Asia-Pacific (APAC) regional instance and store data in a legally appropriate location for where they operate or live. The Veeva eConsent editor uses the region configured for the vault, and MyVeeva for Patients uses the region of the vault from which the eConsent forms are sent.
Additional Enhancements
The following enhancements are now available for MyVeeva for Patients and the Veeva eConsent editor:
| Component | Users | Description | Number |
|---|---|---|---|
| eConsent - Editor | Site Staff, Sponsor Staff | You can now add videos on eConsent forms by clicking the Media icon on the text editor’s toolbar. This functionality was added to the Veeva eConsent editor with the 21R2 release in a limited capacity and is now available to all users. See the Working With Media section in Clinical Operations Help for more information. | MYVC-3642 |