Note Veeva eConsent may be provided by your sponsor for use on a Connected Study or enabled for your SiteVault account as a paid add-on to use across all studies. Contact the Site Success Team to learn more.
Translating an eConsent Form
Complete the following steps to translate an English eConsent form into another language to send to a MyVeeva user.
- Navigate to the Documents tab and select Library.
- Select the English blank informed consent form that you want to translate.
- Select Make a Copy from the actions menu to make a copy.
- You don’t need to change the name of the form. The new document is named automatically when you save.
- Leave the Copy Content and Copy Fields check boxes deselected.
- Select Continue.
- On the Doc Info page for the new document:
- Change the language from English to the language and locale you’re translating the eConsent form to.
- Remove or change the Document Date, if needed.
- Select Save.
- Use the eConsent editor to replace the English text and content of the source document with your desired translated content and Check In the form to SiteVault when finished.
- Complete a review and approval process for the translated document as needed.
- Change the eConsent form’s state to Approved for Use from the actions menu.
- Navigate to the patient record of a participant whose language and locale are the ones you translated the form to. Confirm that the participant’s language and locale are correct.
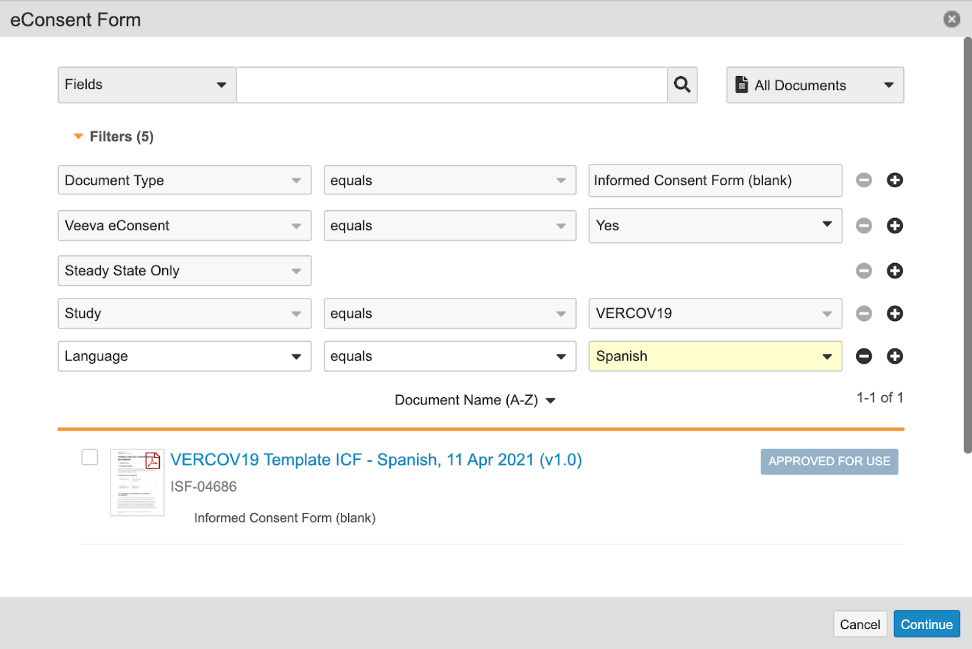
- Navigate to the same patient’s study participant record and select Send eConsent from the actions menu. The resulting dialog will filter to only include eConsent forms available in the correct locale-specific language. The language and locale filters can be removed or modified as needed.
- The participant receives an invitation email or notification with the new form based on the language and locale in their patient record. Note that even if the participant’s language is set to the correct language, if the locale is blank, then the email is sent in English by default.
- When the participant selects the View eConsent link in the email or notification, MyVeeva for Patients is displayed in the locale-specific language set in their browser or device’s language preferences if it is supported.
- Participants can select their preferred language and locale in MyVeeva for Patients once they register an account.
- They can now view and sign the translated eConsent form.
A downloaded .PDF copy of a translated eConsent form will have fields that always appear in English. These fields are:
- The Principal Investigator label
- The page number and date in the footer
Send eConsent Dialog Box
Selecting Send eConsent from the actions menu for a study participant displays a dialog box where you can select one or more eConsent forms to send. If the patient’s language and locale are not blank, the list of eConsent forms are filtered by them. This filter can be modified or removed by expanding the filter section of the dialog box.
 SiteVault
SiteVault