When managing your site’s Site Connect study access, each user must be assigned a role and responsibilities from the lists below.
Site Staff Site Connect Roles
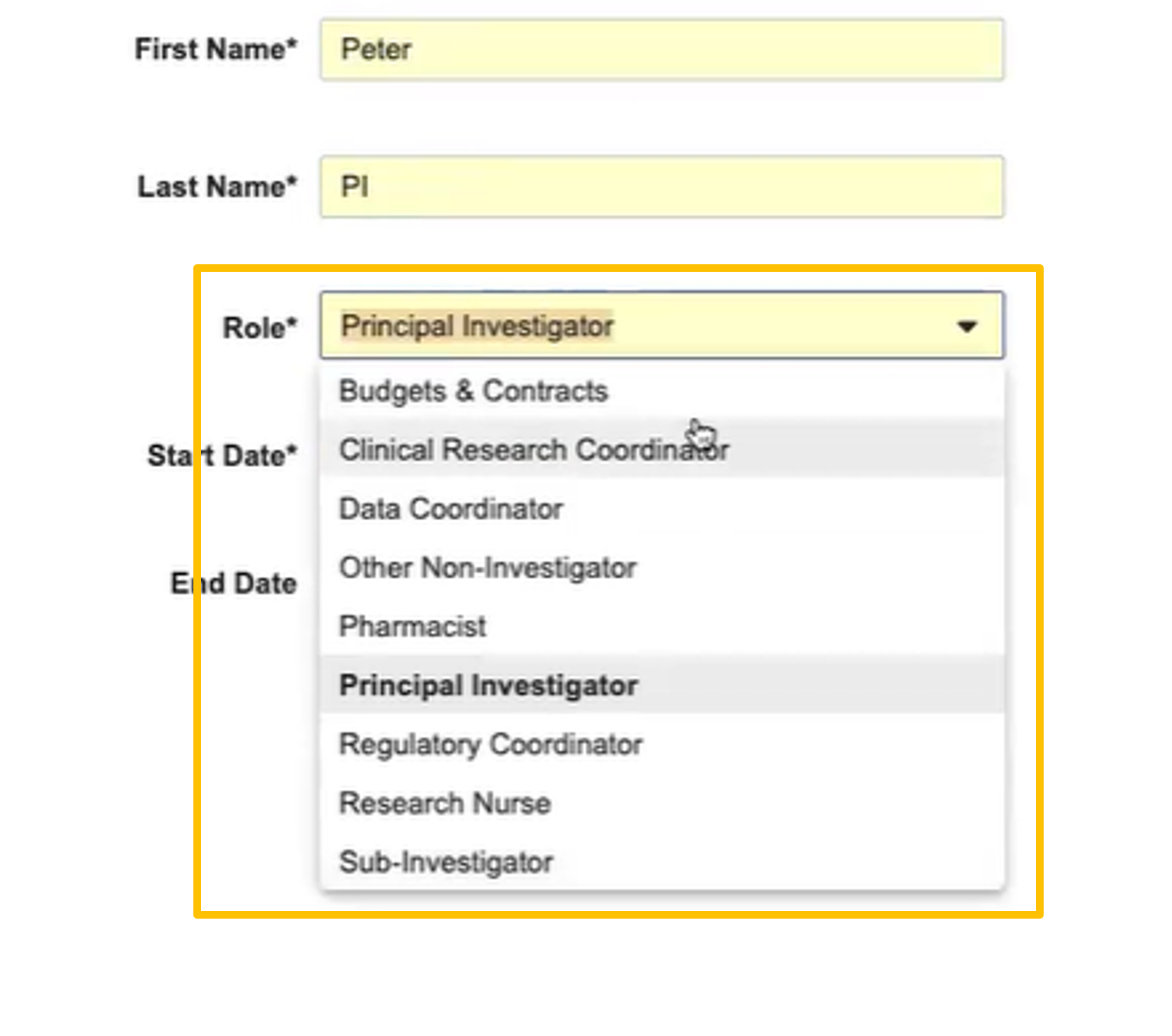
- Budgets & Contracts
- Clinical Research Coordinator
- Data Coordinator
- Other Non-Investigator
- Pharmacist
- Principal Investigator
- Regulatory Coordinator
- Research Nurse
- SubInvestigator
Site Staff Site Connect Responsibilities
When managing users, the list of responsibilities will include items that are not specific to Site Connect as this list is also used to manage study training assignments. Responsibilities that are specific to Site Connect are listed below.
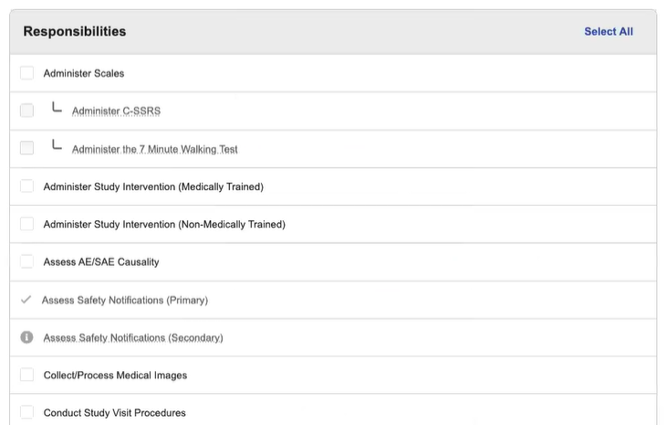
- Assess Safety Notifications (Primary)
- Assess Safety Notifications (Secondary)
- Maintain Essential Documents
- Manage Unblinded Content
- Maintain Site Reimbursement
Please note the following responsibility details:
- In some cases, a Sponsor/CRO may associate specific roles and responsibilities; those associations cannot be removed.
- Document notifications are only sent to site staff with the Maintain Essential Documents responsibility. The following roles automatically receive this responsibility: Principal Investigator, SubInvestigator, Clinical Research Coordinator, and Reg Coordinator.
- Primary or secondary safety recipients are determined by the Assess Safety Notifications (Primary)/(Secondary) responsibilities.