Overview
Veeva eCOA captures questionnaire responses (surveys) directly from clinical trial participants using an app or webpage. It also captures clinical measurements observed by a trained healthcare professional (eClinRO). Veeva eCOA provides a simple access point to manage your participants and review ePRO and eClinRO data and compliance.
Learn more about the different sections in the app below.
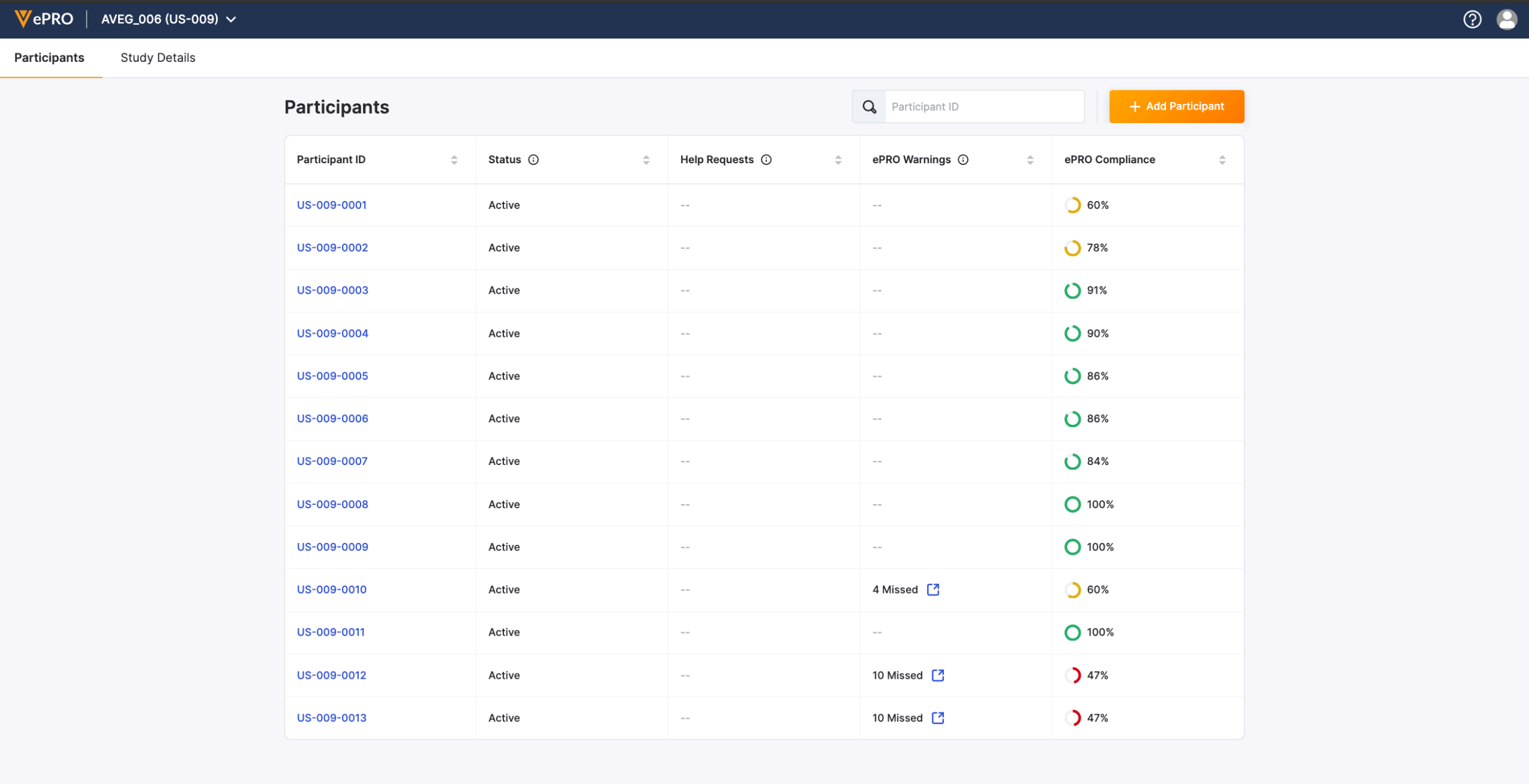
Participants List
All of the participants who are taking part in the study are displayed in the Participants list. You can see a general overview that includes information about compliance and help requests. You can also add new participants from this page.
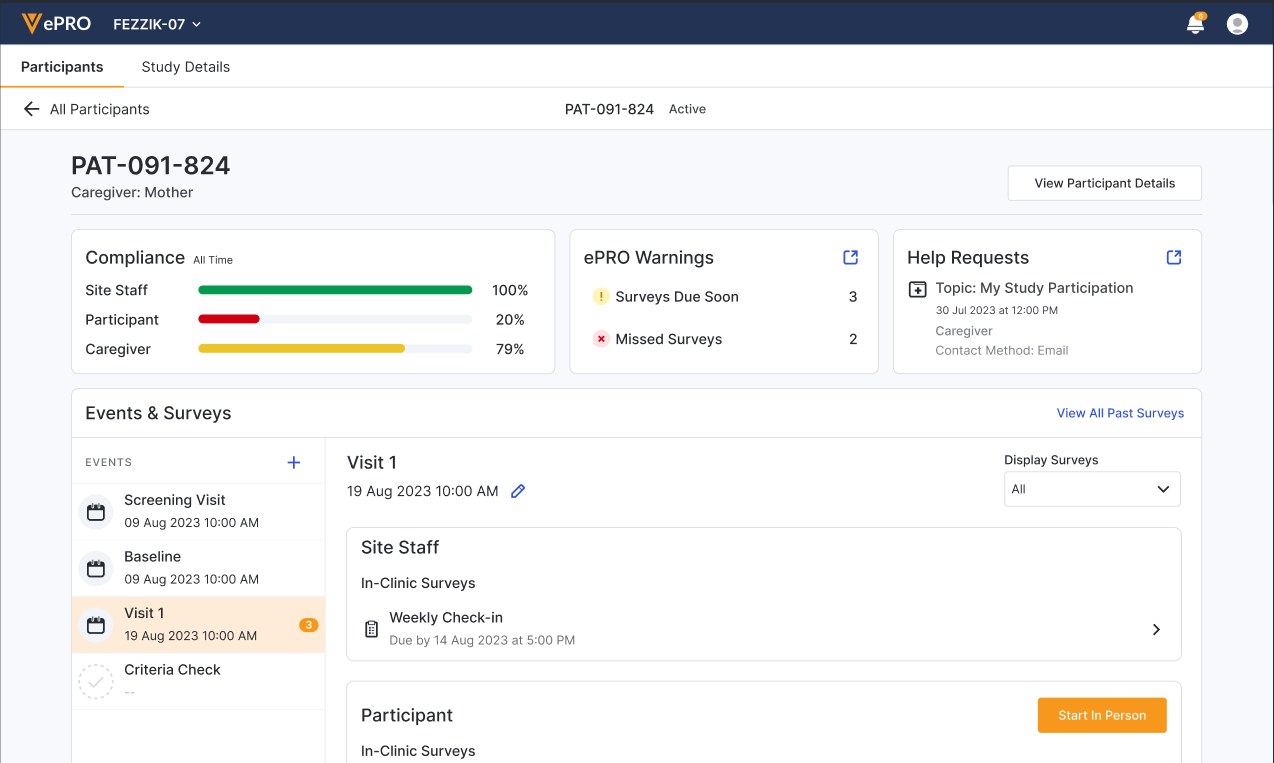
Participant Page
You can see all study information that is related to a specific participant on that participant’s page. You can get an activation code to help them register or reset their account, add event datetimes, review help requests, and even begin in-person surveys from this page.
Study Details
You can activate study versions, connect to a study in SiteVault if applicable, and run reports from the Study Details page.